
5-Bromo-2-fluorophenol synthesis
- Product Name:5-Bromo-2-fluorophenol
- CAS Number:112204-58-7
- Molecular formula:C6H4BrFO
- Molecular Weight:191

112204-57-6

112204-58-7
General procedure for the synthesis of 5-bromo-2-fluorophenol from 5-bromo-2-fluorophenylboronic acid: a tetrahydrofuran (400 mL) solution of 2,2,6,6-tetramethylpiperidine (28 mL, 165 mmol) cooled to -20 °C was treated with n-butyllithium (63 mL of a 2.5 M hexane solution, 157.5 mmol). The mixture was cooled to -78 °C, then 1-bromo-4-fluorobenzene (16.5 mL, 150 mmol) was slowly added dropwise over 10 min and stirring was continued for 3 h at -78 °C. Triisopropyl borate (40 mL, 172.5 mmol) was then added and stirring was continued at -78 °C for 30 min. The cooling bath was removed and when the internal temperature of the reaction rose to -4 °C, 5 N hydrochloric acid (75 mL) was added and the mixture was stirred to ambient temperature. After stirring for 1 h at ambient temperature, most of the tetrahydrofuran was evaporated and the mixture was partitioned between ether (500 mL) and 1 N hydrochloric acid (500 mL). The organic phase was extracted with 2 N sodium hydroxide (400 mL) and the organic phase was discarded. The aqueous phase was cooled in an ice water bath and 5 N hydrochloric acid (150 mL) was added dropwise over 15 min. The resulting white solid was collected and dried under vacuum to give 5-bromo-2-fluorophenylboronic acid (25 g, 76%). A tetrahydrofuran solution of 5-bromo-2-fluorophenylboronic acid (25 g, 114 mmol) was treated with 35 wt% aqueous hydrogen peroxide (7.8 mL) and 4 N aqueous sodium hydroxide (1.4 mL). A mild exothermic reaction raised the internal temperature to 40 °C. The mixture was stirred at ambient temperature for 14 h. Manganese dioxide (200 mg) was then added and stirring was continued for 90 min. The reaction mixture was filtered through GF/filter paper and the filtrate was concentrated on a rotary evaporator. The residue was partitioned between ether (400 mL) and water and the organic phase was washed with water and brine and dried with anhydrous magnesium sulfate. Filtration and evaporation to dryness afforded 5-bromo-2-fluorophenol (19.7 g, 90%) as a colorless liquid. A tetrahydrofuran (50 mL) solution of ice-cooled 5-bromo-2-fluorophenol (1.91 g, 10 mmol) and L-methyl-1H-1 ,2,3-triazol-4-ylmethanol (1.24 g, 11 mmol) was treated with diisopropyl azodicarboxylate (3.03 g, 15 mmol) and triphenylphosphine (3.93 g, 15 mmol) over a period of 10 minutes. The mixture was stirred at ambient temperature for 12 h, then glacial acetic acid (1 mL) was added. The reaction mixture was concentrated under vacuum and the residue was partitioned between ethyl acetate and 0.01 N sodium hydroxide solution. The organic phase was washed with water and brine, dried with anhydrous magnesium sulfate and concentrated to give an oil. Purification was by silica gel chromatography, using a gradient of ethyl acetate (20-50%) eluting with isohexane. The product-containing fraction was concentrated and the residue was ground in isohexane with 10% ether to give 4-(5-bromo-2-fluorophenoxymethyl)-1-methyl-1H-1,2,3-triazole (1.9 g, 66%) as a white solid. Reaction of 2-(8-fluoroimidazo[1,2-a]pyridin-7-yl)propan-2-ol with 4-(5-bromo-2-fluorophenoxymethyl)-1-methyl-1H-1,2,3-triazole afforded 2-{8-fluoro-3-[4-fluoro-3-(1-methyl-1H-1,2,3-triazol-4-ylmethoxy)phenyl]imidazo[1,2-a]pyridin-7-yl} propan-2 -alcohol, as off-white solid.
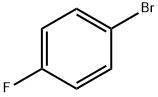
460-00-4
633 suppliers
$6.00/10g

112204-58-7
221 suppliers
$10.00/5g
Yield:112204-58-7 85%
Reaction Conditions:
Stage #1:1-Bromo-4-fluorobenzene with 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -78;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 2.5 h;
Steps:
7A
To a solution of 2,2,6,6-tetramethyl piperidine (5.6 mL, 33.2 mmol) in THF at -20° C. was added n-BuLi (1.6 M in hexanes, 18.8 mL, 30 mmol). The mixture was stirred at -20° C. for 10 min before it was cooled to -78° C. 1-Bromo-4-fluorobenzene (2.95 mL, 27 mmol) was added over 10 min and the mixture was stirred at -78° C. for 2.0 h before trimethyl borate (6.0 mL, 54 mmol) was added. The mixture was stirred at -78° C. for 30 min and then at rt for 2.0 h. After it was cooled back to 0° C., glacial acetic acid (4.86 mL, 81 mmol) was added and stirred for 30 min, followed by addition of 30% H2O2 (4.86 mL, 81 mmol). The mixture was stirred at rt for 24 h, quenched by addition of MnO2 (40 mg). After stirring at rt for 30 min, the cloudy solution was filtered through a pad of wet Celite and extracted with EtOAc. The EtOAc layer was washed with aquous NaHSO3, brine and dried over Na2SO4. The crude residue was purified by flash column chromatography (EtOAc:hexanes=1:5) to give 4.4 g (85%) of 7A as a liquid. 1H NMR (400 MHz, CDCl3) δ ppm 5.39 (s, 1H) 6.90-6.98 (m, 2H) 7.14 (dd, J=8.13, 1.98 Hz, 1H).
References:
Bristol-Myers Squibb Company US2010/227894, 2010, A1 Location in patent:Page/Page column 27

112204-57-6
135 suppliers
$11.00/1g

112204-58-7
221 suppliers
$10.00/5g
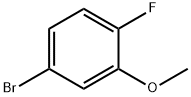
103291-07-2
132 suppliers
$10.00/5g

112204-58-7
221 suppliers
$10.00/5g

92545-83-0
24 suppliers
$106.00/250 mg

112204-58-7
221 suppliers
$10.00/5g

348-61-8
395 suppliers
$6.00/5g

2105-94-4
365 suppliers
$6.00/5g

112204-58-7
221 suppliers
$10.00/5g