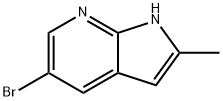
5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine synthesis
- Product Name:5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine
- CAS Number:1111638-02-8
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
![5-Bromo-2-methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1111638-01-7.gif)
1111638-01-7
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
Preparation of Method A Steps for the synthesis of intermediate 4: 5-bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine: Ingredient 3 (88 mg, 0.251 mmol) was dissolved in methanol (4 mL), 2 N sodium hydroxide solution (1 mL) was added, and the reaction mixture was heated and refluxed for 2 hours. After completion of the reaction, ethyl acetate was added for extraction and the organic phase was washed sequentially with 1 N sodium hydroxide solution and water. Purification by silica gel column chromatography (slow gradient elution, volume fraction of methanol in dichloromethane increased from 0% to 2%) afforded the target product 4 (40 mg, 0.19 mmol, 76% yield). Product characterization data were as follows: 1H NMR (CDCl3, 300 MHz): δ 10.26 (br s, 1H), 8.22 (d, J = 2.1 Hz, 1H), 8.92 (d, J = 2.1 Hz, 1H), 6.13 (s, 1H), 2.52 (s, 3H). MS (m/z): 210 (M + H).

1312755-43-3
1 suppliers
inquiry
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
117 suppliers
$19.00/100mg
Yield: 97%
Reaction Conditions:
with potassium tert-butylate in tert-butyl alcohol at 85; for 1 h;
Steps:
Step 3 : 5-Bromo-2-methyl-lH-pyrrolor2,3-b1pyridine5-Bromo-3-prop-l-ynyl-pyridin-2-ylamine (91 g, 431 mmol) was treated with a 1M solution of potassium ie/t-butoxide in ie/t-butanol (700 mL) and the reaction mixture was heated at 85 °C for 1 hour. The mixture was then allowed to cool to ambient temperature and poured onto a 1: 1 mixture of water / ice {ca. 1 L). The resultant precipitate was collected by filtration, washed with water and left to air dry. The resultant solid was dissolved in dichloromethane, dried (Na2S04) and evaporated then triturated with diethyl ether to afford the title compound as a brown solid (88.7 g, 97%). NMR (400 MHz, CDC13): 10.21 (s, 1H), 8.22 (d, J = 2.1 Hz, 1H), 7.91 (d, J = 2.1 Hz, 1H), 6.13 (s, 1H),2.52 (s, 3H).
References:
F. HOFFMANN-LA ROCHE AG;DYKE, Hazel Joan;GAZZARD, Lewis J.;WILLIAMS, Karen WO2011/73263, 2011, A1 Location in patent:Page/Page column 79
![5-Bromo-2-methyl-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1111638-01-7.gif)
1111638-01-7
31 suppliers
$410.00/250mg
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
117 suppliers
$19.00/100mg

183208-35-7
559 suppliers
$5.00/1g
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
117 suppliers
$19.00/100mg
![1H-Pyrrolo[2,3-b]pyridine, 5-bromo-1-(phenylsulfonyl)-](/CAS/GIF/1001070-33-2.gif)
1001070-33-2
79 suppliers
$65.00/100mg
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
117 suppliers
$19.00/100mg

381233-96-1
322 suppliers
$8.00/1g
![5-Bromo-2-methyl-1H-pyrrolo[2,3-b]pyridine](/CAS2/GIF/1111638-02-8.gif)
1111638-02-8
117 suppliers
$19.00/100mg