
5-Chloroindole-3-carboxylic acid synthesis
- Product Name:5-Chloroindole-3-carboxylic acid
- CAS Number:10406-05-0
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6

17422-32-1

407-25-0

10406-05-0
5.0 g (33.0 mmol) of commercially available 5-chloro-1H-indole was dissolved in 50 mL of anhydrous N,N-dimethylformamide (DMF) and the reaction system was kept at 0°C. Slowly 7.35 g (35.0 mmol) of trifluoroacetic anhydride was added dropwise to the above solution. After the dropwise addition, the reaction mixture was stirred at room temperature for 3 hours. After completion of the reaction, the mixture was slowly poured into 200 mL of ice water and the precipitate was collected by filtration. The resulting precipitate was suspended in 200 mL of 20% sodium hydroxide (NaOH) aqueous solution and heated at reflux overnight. After cooling, the aqueous phase was extracted twice with dichloromethane (100 mL each time). The organic phases were combined and the aqueous phase was acidified to pH 2-3 with concentrated hydrochloric acid to precipitate a solid. The crystalline product was collected by filtration and washed with a small amount of cold water. Finally, the product was dried in a vacuum drying oven to give 6.0 g of 5-chloroindole-3-carboxylic acid in 93% yield.

17422-32-1
376 suppliers
$6.00/1g

407-25-0
472 suppliers
$9.00/1g

10406-05-0
129 suppliers
$45.00/100mg
Yield: 93%
Reaction Conditions:
Stage #1:5-chloro-1H-indole;trifluoroacetic anhydride in N,N-dimethyl-formamide at 0 - 20; for 3 h;
Stage #2: with sodium hydroxide in waterHeating / reflux;
Steps:
59.A
A solution of 5.0 g (33.0 mmol) of commercially available 5-chloro-1H-indole in 50 ml dry DMF was kept at 0° C., while 7.35 g (35.0 mmol) trifluoroacetanhydride was added dropwise. After 3 h stirring at room temperature the mixture was poured into 200 ml water, and the precipitate was filtered with suction and heated with reflux overnight in 200 ml 20% NaOH. It was extracted twice with dichloromethane, and the aqueous layer was acidified with hydrochloric acid. The crystalline title compound was collected by filtration and dried in vacuo. yield: 6.0 g (93%)
References:
Takeuchi, Kumiko;Jirousek, Michael Robert;Paal, Michael;Ruhter, Gerd;Schotten, Theo US2004/9976, 2004, A1 Location in patent:Page/Page column 52
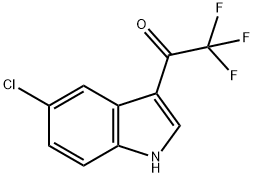
1419517-55-7
6 suppliers
inquiry

10406-05-0
129 suppliers
$45.00/100mg

10406-06-1
163 suppliers
$6.00/100mg

10406-05-0
129 suppliers
$45.00/100mg

17422-32-1
376 suppliers
$6.00/1g

10406-05-0
129 suppliers
$45.00/100mg

827-01-0
157 suppliers
$9.00/250mg

10406-05-0
129 suppliers
$45.00/100mg