
6,7-Bis-(2-methoxyethoxy)-4(3H)-quinazolinone synthesis
- Product Name:6,7-Bis-(2-methoxyethoxy)-4(3H)-quinazolinone
- CAS Number:179688-29-0
- Molecular formula:C14H18N2O5
- Molecular Weight:294.3
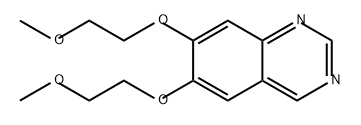
1208902-93-5

179688-29-0
GENERAL METHODS: To a solution of 6,7-dimethoxyquinazoline (200.0 mg, 1.05 mmol) in anhydrous ethanol (20 mL) was sequentially added 40% peracetic acid (1.0 mL, 5.26 mmol) and concentrated sulfuric acid (0.01 mL, 1.8 mmol). The reaction mixture was stirred in an oil bath at 60 °C for 4-12 h (the progress of the reaction was monitored by TLC). Upon completion of the reaction, the system was cooled to room temperature and residual peroxides were quenched by the addition of excess sodium bisulfite (541.8 mg, 5.26 mmol). After continued stirring for 20 min, the insoluble material was removed by filtration and the filtrate was concentrated under reduced pressure to give the crude product. The crude product was washed with a solvent mixture of ethanol and petroleum ether (1:1, v/v) to afford 6,7-dimethoxyethoxyquinazolin-4-one (179.7 mg, 83% yield) as a light yellow solid with a melting point of >300°C. The crude product was washed with a solvent mixture of ethanol and petroleum ether (1:1, v/v) to afford 6,7-dimethoxyethoxyquinazolin-4-one as a light yellow solid.

3473-63-0
513 suppliers
$5.00/25g

179688-27-8
147 suppliers
$31.00/250mg

179688-29-0
463 suppliers
$6.00/1g
Yield:179688-29-0 98%
Reaction Conditions:
Stage #1: formamidine acetic acid;ethyl 2-amino-4,5-bis(2-methoxyethoxy)-benzoate in butan-1-ol at 80; for 5 h;Inert atmosphere;
Stage #2: in Isopropyl acetate; for 1 h;Reflux;
Steps:
5 E. Synthesis of Compound (vi) the
Compound (v) (171.1g), formamidine acetate (79.8 g) was dissolved in n-butanol (1027mL), and nitrogen at elevatedTemperature to 80 ° C for 5 hours, the reaction was complete by HPLC. Spin dry n-butanol, was added 1711g of isopropyl acetate heated to reflux for 1Hours, cooled to _5 ° C for 4 hours. Filtered, 50 ° C and dried under blast (vi) (164.0g), yield 98%, purity>95%. NMR data identified compound (vi) as follows
References:
CN103709110,2016,B Location in patent:Paragraph 0067; 0068

1208902-93-5
0 suppliers
inquiry

179688-29-0
463 suppliers
$6.00/1g

60100-09-6
0 suppliers
inquiry

179688-27-8
147 suppliers
$31.00/250mg

179688-29-0
463 suppliers
$6.00/1g

179688-27-8
147 suppliers
$31.00/250mg

149-73-5
551 suppliers
$12.00/5g

179688-29-0
463 suppliers
$6.00/1g

179688-27-8
147 suppliers
$31.00/250mg

179688-29-0
463 suppliers
$6.00/1g