
6-AMIDINO-2-NAPHTHOL METHANESULFONATE synthesis
- Product Name:6-AMIDINO-2-NAPHTHOL METHANESULFONATE
- CAS Number:82957-06-0
- Molecular formula:C12H14N2O4S
- Molecular Weight:282.32

52927-22-7
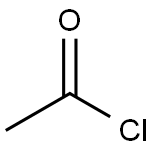
75-36-5

82957-06-0
1. Add 800 mL of anhydrous ethanol to a dry triple-necked flask, initiate stirring and cool the reaction system to 0-5 °C. 2. 430 mL of acetyl chloride was slowly added dropwise, and the rate of dropwise acceleration was controlled to maintain the reaction temperature at 0-5 °C. 3. 3. after the dropwise addition, keep the reaction system at 0-5°C for a period of time. 4. 85 g of 6-cyano-2-naphthol was added to the reaction system, warmed to 10 °C and kept for 10 hours. 5. After completion of the reaction, methyl tert-butyl ether was added, stirred for 1 hour and filtered. 6. The filter cake was washed with methyl tert-butyl ether, dried and mixed with ethanol. 7. The mixture was heated to 50°C and bubbled with dry ammonia for 3 hours. 8. 8. At the end of the reaction, the solvent was removed by evaporation under reduced pressure to give a yellow solid. 9. The solid was stirred with saturated sodium bicarbonate solution for a period of time, filtered and washed with water to neutral. 10. 10. Suspend the product in methanol and add appropriate amount of methanesulfonic acid solution dropwise. 11. 11. Add methyl tert-butyl ether to precipitate the solid, filter and wash with methyl tert-butyl ether. 12. Recrystallization was carried out with ethanol to give 75 g of a yellow solid product with a melting point of 227-230 °C and a yield of 53%.

52927-22-7
301 suppliers
$6.00/1g

75-36-5
588 suppliers
$17.92/100G

82957-06-0
222 suppliers
$75.00/100mg
Yield:82957-06-0 53%
Reaction Conditions:
Stage #1:6-cyano-2-naphthol;acetyl chloride in ethanol;tert-butyl methyl ether at 0 - 50; for 10 h;Pinner Imino Ether Synthesis;
Stage #2: with methanesulfonic acid in methanol;tert-butyl methyl etherSolvent;
Steps:
3 Example 3:
Take 800 ml of absolute ethanol in three bottles,The mixture was cooled to 0-5 ° C with stirring and 430 ml of acetyl chloride was added dropwise.Dropping completed, at 0 ~ 5 for a period of time, put 6-cyano-2-naphthol 85 g, 10 ° C for 10 hours, methyl tert-butyl ether was added and stirred for 1 hour to filter,The filter cake was washed with methyl tert-butyl ether, dried and mixed directly with ethanol, heated to 50 ,Dry ammonia was bubbled for 3 hours and evaporated to dryness under reduced pressure to give a yellow solid.The mixture was stirred with saturated sodium bicarbonate solution for a period of time, filtered, washed with water until neutral.Wet products suspended in methanol, dropping a certain amount of methanesulfonic acid solution,Methyl tert-butyl ether was added to precipitate a solid, which was filtered, washed with methyl tert-butyl ether,Recrystallization from ethanol gave 75 g of a yellow solid,The temperature of 227 ° C ~ 230 ° C,Yield 53%.
References:
Beijing Lanbeiwang Bio-pharmaceutical Technology Co., Ltd.;Wan Liuyi;Fu Jifeng;Li Shuming CN103896809, 2017, B Location in patent:Paragraph 0010; 0011; 0012; 0013

52927-22-7
301 suppliers
$6.00/1g

82957-06-0
222 suppliers
$75.00/100mg

765871-54-3
17 suppliers
inquiry

82957-06-0
222 suppliers
$75.00/100mg

75-75-2
649 suppliers
$10.00/5g

58200-88-7
14 suppliers
inquiry

82957-06-0
222 suppliers
$75.00/100mg