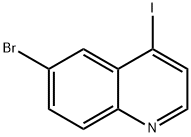
6-BROMO-4-IODOQUINOLINE synthesis
- Product Name:6-BROMO-4-IODOQUINOLINE
- CAS Number:927801-23-8
- Molecular formula:C9H5BrIN
- Molecular Weight:333.95

1086062-75-0

927801-23-8
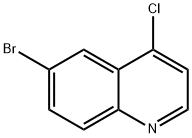
4.1.9 Synthesis of 6-bromo-4-iodoquinoline (12): To an anhydrous ethyl acetate (20 mL) solution of 6-bromo-4-chloroquinoline (11) (3.50 g, 14.46 mmol) was added hydrochloric acid saturated ethyl acetate (40 mL), and the formation of a white precipitate was immediately observed. After stirring for 30 min, the suspension was concentrated under vacuum to give 6-bromo-4-chloroquinoline hydrochloride as an off-white solid (3.91 g, 14.14 mmol). Subsequently, 6-bromo-4-chloroquinoline hydrochloride (3.91 g, 14.14 mmol), anhydrous potassium iodide (9.76 g, 70.70 mmol), and anhydrous acetonitrile (100 mL) were added in a two-neck flask. The resulting slurry was stirred under reflux conditions for 48 hours and then cooled to room temperature. The reaction mixture was treated with saturated aqueous sodium bicarbonate solution (40 mL) and 5% sodium sulfite solution (20 mL). The reaction mixture was extracted with dichloromethane (200 mL x 2), and the combined organic phases were dried over magnesium sulfate and concentrated under vacuum to give the crude product. The crude product was further purified by silica gel column chromatography (25% ethyl acetate/petroleum ether) to afford the target compound 6-bromo-4-iodoquinoline (4.42 g, 13.27 mmol, 94% yield) as an off-white solid. 1H NMR (500 MHz, DMSO-d6) δ 8.51 (d, J = 4.5 Hz, 1H, Ar-H), 8.21 (d, J = 4.5 Hz, 1H, Ar-H), 8.11 (t, J = 1.5 Hz, 1H, Ar-H), 7.97-7.91 (m, 2H, Ar-H). ESI-MS: m/z = 334 [M + H]+.

1086062-75-0
1 suppliers
inquiry

927801-23-8
72 suppliers
$32.00/250mg
Yield: 94%
Reaction Conditions:
with potassium iodide in acetonitrile for 48 h;Reflux;
Steps:
9 4.1.9. 6-Bromo-4-iodoquinoline (12)
4.1.9
6-Bromo-4-iodoquinoline (12)
To a solution of 6-bromo-4-chloroquinoline (11) (3.50 g, 14.46 mmol) in anhydrous EtOAc (20 mL) was added HCl-saturated EtOAc (40 mL) and a white precipitate formed immediately.
After stirring for 30 min, the suspension was concentrated under vacuum to afford 6-bromo-4-chloroquinoline hydrochloride as an off white solid (3.91 g, 14.14 mmol). A two-neck flask was charged with 6-bromo-4-chloroquinoline hydrochloride (3.91 g, 14.14 mmol), anhydrous potassium iodide (9.76 g, 70.70 mmol) and anhydrous acetonitrile (100 mL).
The resulting slurry was stirred at reflux for 48 h and allowed to cool to room temperature.
Saturated aqueous NaHCO3 solution (40 mL) was added to the mixture, followed by 20 mL of a 5% sodium sulfite solution.
The reaction mixture was extracted with CH2Cl2 (200 mL * 2).
The combined organic extracts were dried over magnesium sulfate and concentrated in vacuo to give the crude product, which was further purified by silica gel column chromatography (25% ethyl acetate/petroleum ether) to give the title compound (4.42 g, 13.27 mmol, 94% yield) as an off-white solid. 1H NMR (500 MHz, DMSO-d6) δ 8.51 (d, J = 4.5 Hz, 1H, Ar-H), 8.21 (d, J = 4.5 Hz, 1H, Ar-H), 8.11 (t, J = 1.5 Hz, 1H, Ar-H), 7.97-7.91 (m, 2H, Ar-H). ESI-MS: m/z = 334 [M+H]+.
References:
Lv, Xiaoqing;Ying, Huazhou;Ma, Xiaodong;Qiu, Ni;Wu, Peng;Yang, Bo;Hu, Yongzhou [European Journal of Medicinal Chemistry,2015,vol. 99,art. no. 7904,p. 36 - 50]
65340-70-7
272 suppliers
$7.00/250mg

927801-23-8
72 suppliers
$32.00/250mg
![5-[(4-Bromophenylamino)methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione ,98%](/CAS2/GIF/187278-01-9.gif)
187278-01-9
21 suppliers
$110.00/500mg

927801-23-8
72 suppliers
$32.00/250mg

106-40-1
480 suppliers
$12.00/5g

927801-23-8
72 suppliers
$32.00/250mg

145369-94-4
221 suppliers
$10.00/250mg

927801-23-8
72 suppliers
$32.00/250mg