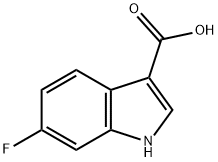
6-FLUORO-1H-INDOLE-3-CARBOXYLIC ACID synthesis
- Product Name:6-FLUORO-1H-INDOLE-3-CARBOXYLIC ACID
- CAS Number:23077-44-3
- Molecular formula:C9H6FNO2
- Molecular Weight:179.15

399-51-9

407-25-0

23077-44-3
Trifluoroacetic anhydride (9.009 mL, 64.8 mmol, 2.2 eq.) was added slowly and dropwise to a stirred solution of 6-fluoroindole (4.000 g, 29.6 mmol, 1.0 eq.) dissolved in DMF (25 mL) at 0 °C (temperature controlled using an external ice bath). After the reaction mixture was kept in the ice bath for 3 h, the reaction was quenched with 40 mL of 2N sodium carbonate solution. The precipitate was collected by filtration and washed with distilled water. The resulting solid was dissolved in 4 M NaOH solution (50 mL) and refluxed for 4 hours. After completion of the reaction, the mixture was cooled to room temperature and poured into 50 mL of distilled water. The mixture was cooled again in an ice bath and the pH was adjusted slowly by adding 6N HCl to 3. The precipitate precipitated was collected by filtration, washed with distilled water and dried under vacuum to give the off-white solid product 6-fluoro-1H-indole-3-carboxylic acid (3.5 g, 66% yield).

399-51-9
355 suppliers
$11.00/5g

407-25-0
473 suppliers
$9.00/1g

23077-44-3
77 suppliers
$37.00/100mg
Yield: 66%
Reaction Conditions:
Stage #1:6-fluoro-1H-indole;trifluoroacetic anhydride in N,N-dimethyl-formamide at 0; for 3 h;
Stage #2: with sodium hydroxide in N,N-dimethyl-formamide; pH=3 for 4 h;Reflux;
Steps:
1 6-fluoroiindole-3-carboxylic acid (2)
Trifluoroacetic anhydride (9.009 ml, 64.8 mmol, 2.2 equiv.) was added drop wise to a stirring solution of 6-fluoroindole (4.000 g, 29.6 mmol, 1.0 equiv.) in DMF (25 mL) at 0°C (external ice bath temperature). After 3 hours in the ice bath the mixture was quenched with 40 mL of 2N sodium carbonate. The precipitate was collected by filtration and washed with water. The solid was taken up in 4M NaOH (50 mL) and refluxed for 4 hours. The reaction mixture was cooled to room temperature and poured into 50 mL of water. The mixture was cooled in an ice bath and slowly brought to pH 3 with 6N HCl. The precipitate which formed was collected by filtration, washed with water and dried under vacuum to give an off -white solid (3.5 g, 66%). 1H NMR (DMSO-d6, 300 MHz): 12.03 (bs, 1H), 11.87 (s, 1H), 7.99-7.92(m, 2H), 7.23 (dd, J=9.9 & 2.4 Hz, 1Η),7.03-6.97 (m, 1H).
References:
BUCK INSTITUTE FOR RESEARCH ON AGING;JOHN, Varghese;BREDESEN, Dale, E.;SPILMAN, Patricia, R.;JAGODZINSKA, Barbara WO2017/197177, 2017, A1 Location in patent:Paragraph 0219-0221

399-51-9
355 suppliers
$11.00/5g

23077-44-3
77 suppliers
$37.00/100mg
2795-41-7
170 suppliers
$7.00/250mg

23077-44-3
77 suppliers
$37.00/100mg

1043601-53-1
28 suppliers
inquiry

23077-44-3
77 suppliers
$37.00/100mg

1261025-10-8
6 suppliers
inquiry

23077-44-3
77 suppliers
$37.00/100mg