
6-Iodoquinoline synthesis
- Product Name:6-Iodoquinoline
- CAS Number:13327-31-6
- Molecular formula:C9H6IN
- Molecular Weight:255.06
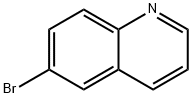
5332-25-2

13327-31-6
General procedure for the synthesis of 6-iodoquinoline from 6-bromoquinoline: sodium iodide (4.32 g, 28.8 mmol), copper(I) iodide (137 mg, 0.72 mmol), N,N'-dimethyl-cyclohexane-1,2-diamine (0.227 mL, 1.44 mmol) and 6-bromoquinoline (3 g, 14.4 mmol) in a solution of dioxane (15 mL). The reaction tube was flushed with nitrogen and sealed with a PTFE septum. Nitrogen was bubbled into the solution through a needle for 10 min to ensure that gas escaped through the needle. After removing the needle, the reaction mixture was stirred at 110°C for 15 hours. Upon completion of the reaction, the green suspension was cooled to room temperature, poured into ice water and extracted with dichloromethane. The organic layer was collected, dried with anhydrous magnesium sulfate, filtered and concentrated in vacuum. The crude product was purified by silica gel column chromatography, eluting sequentially with 100% dichloromethane and dichloromethane/methanol (95:5, v/v) to afford 3.56 g (97% yield) of 6-iodoquinoline as a light yellow solid.1H-NMR (DMSO-d6) δ: 8.93 (1H, dd, J = 1.5, 4.1 Hz), 8.47 (1H, d, J = 2.0 Hz ), 8.33 (1H, d, J = 8.6 Hz), 8.02 (1H, dd, J = 2.0, 8.6 Hz), 7.80 (1H, d, J = 8.6 Hz), 7.56 (1H, dd, J = 4.1, 8.6 Hz).

580-15-4
312 suppliers
$6.00/1g

13327-31-6
190 suppliers
$8.00/100mg
Yield:13327-31-6 79%
Reaction Conditions:
Stage #1:6-aminoquinoline with trifluorormethanesulfonic acid;sodium nitrite in hexane;dimethyl sulfoxide at 5 - 20; for 1 h;
Stage #2: with potassium iodide in hexane;water;dimethyl sulfoxide at 20; for 0.166667 h;
Steps:
Diazotization-Iodination of 3- and 6-Quinoline Amines 4b,c; GeneralProcedure
General procedure: To a solution of hexane (5 mL), DMSO (0.5 mL), and trifluoromethanesulfonicacid (0.54 mL, 6 mmol) at 5 °C were sequentially added thequinoline amine 4b or 4c (2 mmol) and NaNO2 (350 mg, 5 mmol) understirring, and the mixture was stirred for 10 min. The resultingmixture was then stirred for 50 min at r.t. until the starting aminehad been consumed as monitored by TLC. An emission of N2 bubbleswas not observed and the reaction solution gave the positive probeon diazonium salts with β-naphthol. Next, KI (2.4 mmol) in H2O (0.5mL) was added and the mixture was stirred 10 min at r.t. until evolutionof N2 bubbles ceased. In the case of 4b, the solid 3-iodoquinoline6 obtained was filtered, washed with H2O, and dried. In the case of 3c,the reaction mixture was poured into H2O and the oily product 7 was extracted with EtOAc (2 × 25 mL). The combined organic extractswere dried (Na2SO4), filtered, and the solvent was removed under reducedpressure on a rotary evaporator. The product 7 was purified bysilica gel flash chromatography (eluent: CH2Cl2).
References:
Kassanova, Assiya Zh.;Krasnokutskaya, Elena A.;Beisembai, Perizat S.;Filimonov, Victor D. [Synthesis,2016,vol. 48,# 2,p. 256 - 262]

5332-25-2
417 suppliers
$6.00/5g

13327-31-6
190 suppliers
$8.00/100mg
3054-95-3
208 suppliers
$28.00/25g

540-37-4
460 suppliers
$6.00/5g

13327-31-6
190 suppliers
$8.00/100mg
5122-99-6
239 suppliers
$6.00/250mg

13327-31-6
190 suppliers
$8.00/100mg

540-37-4
460 suppliers
$6.00/5g

13327-31-6
190 suppliers
$8.00/100mg