
6-Methoxy-2-vinylnaphthalene synthesis
- Product Name:6-Methoxy-2-vinylnaphthalene
- CAS Number:63444-51-9
- Molecular formula:C13H12O
- Molecular Weight:184.23

2768-02-7
406 suppliers
$10.00/5g

5111-65-9
356 suppliers
$6.00/5g

63444-51-9
120 suppliers
$135.00/1g
Yield: 84%
Reaction Conditions:
with (1,2-dimethoxyethane)dichloronickel(II);18-crown-6 ether;potassium methanolate in N,N-dimethyl-formamide at 35; for 12 h;Inert atmosphere;Hiyama Coupling;Reagent/catalyst;Solvent;
Steps:
General Procedure 1
General procedure: In a nitrogen-filled glove box, aromatic halide (0.2 mmol, 1.0equiv), 18-crown-6 (121.0 mg, 0.46 mmol, 2.3 equiv), KOMe(32.2 mg, 0.46 mmol, 2.3 equiv), NiCl2(glyme) (4.4 mg, 0.02mmol, 10 mol%), and DMF (1.0 mL) were charged to an 8 mL vialequipped with a magnetic stirrer bar. The vinyltrimethoxysilane(68.0 mg, 0.46 mmol, 2.3 equiv) was added. The vial wasremoved from the glove box, and the reaction mixture wasstirred at rt (35 °C) for 12 h. The reaction mixture was thendiluted with EtOAc and washed with water. The organic phasewas dried over Na2SO4, filtered, and concentrated, and theresidue was purified by column chromatography on silica gel togive the product.2-Methoxy-6-vinylnaphthalene (3a)Using general procedure 1: white solid, 31.0 mg, yield: 84%. 1HNMR (400 MHz, CDCl3): = 7.78-7.66 (m, 3 H), 7.62 (dd, J = 8.7,1.7 Hz, 1 H), 7.19-7.10 (m, 2 H), 6.87 (dd, J = 17.6, 10.9 Hz, 1 H),5.84 (dd, J = 17.6, 0.9 Hz, 1 H), 5.30 (dd, J = 10.9, 0.9 Hz, 1 H),3.93 (s, 3 H).
References:
Wei, Shichao;Mao, Yongjun;Shi, Shi-Liang [Synlett,2021,art. no. ST-2020-K0612-C] Location in patent:supporting information
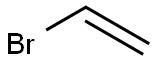
593-60-2
144 suppliers
$25.00/25g

5111-65-9
356 suppliers
$6.00/5g

63444-51-9
120 suppliers
$135.00/1g

74-85-1
113 suppliers
$79.00/11l

5111-65-9
356 suppliers
$6.00/5g

63444-51-9
120 suppliers
$135.00/1g

129113-00-4
44 suppliers
$41.10/1g

63444-51-9
120 suppliers
$135.00/1g

2627-95-4
334 suppliers
$16.80/25G

5111-65-9
356 suppliers
$6.00/5g

63444-51-9
120 suppliers
$135.00/1g