
Methyl 1-amino-1-cyclopentanecarboxylate hydrochloride synthesis
- Product Name:Methyl 1-amino-1-cyclopentanecarboxylate hydrochloride
- CAS Number:60421-23-0
- Molecular formula:C7H14ClNO2
- Molecular Weight:179.64

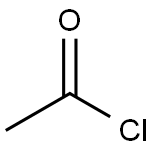
67-56-1
52-52-8

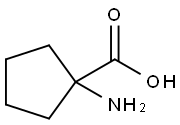
60421-23-0
1. cool a suspension of 1-aminocyclopentanecarboxylic acid (675 g, 5.23 mol, 1.0 eq.) in methanol (6.5 L) at -15 °C using an ice/methanol bath. 2. thionyl chloride (687 mL, 9.4 mol, 1.8 eq.) was slowly added dropwise with controlled titration rate to maintain reaction temperature. 3. after completion of the dropwise addition, the cooling device was removed and the reaction mixture was allowed to warm to room temperature and stirred overnight. 4. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure. 5. 5. The concentrated residue was treated with dichloromethane (1 L) and concentrated again under reduced pressure to give methyl 1-aminocyclopentanecarboxylate hydrochloride as a white solid (938 g, 100% yield). 6. The product was analyzed by 1H NMR. 6. The product was characterized by 1H NMR (CD3OD) and NMR (DMSO-d6) to confirm the correct structure. 7. The product obtained did not require further purification and could be used directly in the subsequent reaction steps.
Yield: 100%
Reaction Conditions:
with thionyl chloride in methanol at -15 - 20;
Steps:
B1c.1 Step 1
To a suspension of 1-aminocyclopentanecarboxylic acid, (675 g, 5.23 mol, 1.0 equiv.) in MeOH (6.5 L) held at -15° C. with an ice/MeOH bath was added SOCl2 (687 mL, 9.4 mol, 1.8 equiv.), dropwise at such a rate that the reaction temp. did not exceed 7° C. After the addition was complete, cooling was removed, the reaction was allowed to stir at room temp. overnight, then was concentrated under reduced pressure. The residue was treated with CH2Cl2 (1 L) and concentrated under reduced pressure to afford methyl 1-aminocyclopentanecarboxylate HCl salt as a white solid (938 g, 100%): 1H NMR (CD3OD) d 1.87-1.94 (m, 8H), 3.83 (s, 3H); NMR (DMSO-d6) δ1.67-1.71 (m, 2H), 1.83-1.98 (m, 4H), 2.06-2.14 (m, 2H), 3.73 (s, 3H), 8.81 (br s 3). This material was used in the next step without further purification
References:
Bayer Corporation US6353006, 2002, B1 Location in patent:Page column 31

52-52-8
301 suppliers
$8.00/1g

60421-23-0
155 suppliers
$21.00/1g
![1-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopentanecarboxylic acid methyl ester](/CAS/20180703/GIF/248262-96-6.gif)
248262-96-6
6 suppliers
inquiry
![Carbamic acid, N-[1-(1-hydroxy-1-methylethyl)cyclopentyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1001426-16-9.gif)
1001426-16-9
0 suppliers
inquiry

1001426-17-0
0 suppliers
inquiry

60421-23-0
155 suppliers
$21.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)