
8-CHLORO-2-PHENYLQUINOLINE-4-CARBOXYLIC ACID synthesis
- Product Name:8-CHLORO-2-PHENYLQUINOLINE-4-CARBOXYLIC ACID
- CAS Number:181048-56-6
- Molecular formula:C16H10ClNO2
- Molecular Weight:283.71
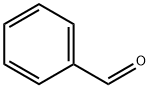
100-52-7
975 suppliers
$20.37/250g

127-17-3
559 suppliers
$5.00/10g

95-51-2
396 suppliers
$5.00/5G

181048-56-6
19 suppliers
$57.50/250mg
Yield:181048-56-6 36%
Reaction Conditions:
Stage #1: benzaldehyde;o-chloroanilinewith borane-THF in acetonitrile at 65; for 0.166667 h;
Stage #2: 2-oxo-propionic acid in acetonitrile; for 24 h;
Steps:
A21 Example A21
Production of 8-chloro-2-phenylquinoline-4-carboxylic acid
To a solution of 2-chloroaniline (0.522 g, 4.09 mmol) in acetonitrile (2.3 mL), benzaldehyde (0.482 g, 4.54 mmol) and boron trifluoride-tetrahydrofuran complex (125 μL, 1.14 mmol) were added, and the mixture was heated to 65° C. 10 minutes later, a solution of pyruvic acid (0.200 g, 2.27 mmol) in acetonitrile (3.8 mL) was added dropwise thereto over 3 hours. 21 hours later, the reaction solution was cooled to room temperature, and saturated aqueous sodium chloride solution was added thereto, followed by extraction with tetrahydrofuran.
The organic layer was washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and then filtered, and the solvent was distilled off under reduced pressure.
The residue was purified by silica gel column chromatography (chloroform/methanol).
The obtained crude product was suspended and washed with chloroform and dried under reduced pressure to obtain the title compound (0.232 g, yield: 36%) as a faint yellow solid.
1H-NMR (400 MHz, DMSO-D6) δ: 14.2 (1H, s), 8.61 (1H, dd, J=8.5, 1.2 Hz), 8.58 (1H, s), 8.40-8.38 (2H, m), 8.07 (1H, dd, J=7.3, 1.2 Hz), 7.68 (1H, dd, J=7.3, 1.2 Hz), 7.64-7.55 (3H, m)
ESI-MS (m/z): 284, 286 (M+H)+
References:
US2022/169640,2022,A1 Location in patent:Paragraph 0201-0203

7477-63-6
191 suppliers
$6.00/1g
98-86-2
796 suppliers
$9.00/5g

181048-56-6
19 suppliers
$57.50/250mg

127-17-3
559 suppliers
$5.00/10g

95-51-2
396 suppliers
$5.00/5G

181048-56-6
19 suppliers
$57.50/250mg
