
[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:850363-42-7
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19
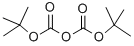
24424-99-5

24358-62-1
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
General procedure for the synthesis of tert-butyl (1-(4-bromophenyl)ethyl)carbamate from di-tert-butyl dicarbonate and 1-(4-bromophenyl)ethylamine (refer to Example 5): synthesis of 1-bromo-4-(1-tert-butoxycarbonylaminoethyl)benzene (refer to compound 5). Triethylamine (2.09 mL) was added to a solution of 2.00 g (10.0 mmol) of 1-(4-bromophenyl)ethylamine in 22.3 mL of dichloromethane and the mixture was cooled in an ice bath. Under argon protection and stirring, 2.87 mL (12.0 mmol) of di-tert-butyl dicarbonate was slowly added to the mixture. Subsequently, the reaction mixture was warmed to room temperature and stirring was continued for 1 hour. Upon completion of the reaction, the reaction solution was poured into 200 mL of water and extracted with 200 mL of chloroform. The organic layer was washed sequentially with water, saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate and concentrated under vacuum. The resulting solid was washed twice with 10 mL of hexane to give 2.71 g of tert-butyl (1-(4-bromophenyl)ethyl)carbamate as a white powder (yield: 90%). rf value: 0.55 (n-hexane: ethyl acetate=4:1, v/v). Mass spectrum (FAB, m/z): 300, 302 (M++1). 1H-NMR spectrum (DMSO-d6, δ ppm): 1.27 (d, J=6.8 Hz, 3H), 1.36 (brs, 9H), 4.51-4.64 (m, 1H), 7.25 (d, J=8.3 Hz, 2H), 7.35-7.45 (m, 1H) , 7.49 (d, J=8.3 Hz, 2H).

24424-99-5
857 suppliers
$13.50/25G

24358-62-1
157 suppliers
$9.00/1g
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
49 suppliers
$45.00/50mg
Yield:850363-42-7 90%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1 h;
Steps:
5
(Referential Example 5) Synthesis of 1-bromo-4-(1-tert-butoxycarbonylamino-ethyl)benzene (referential compound 5) Triethylamine (2.09 ml) was added to a solution of 2.00 g (10.0 mmol) of 4-(1-aminoethyl)-1-bromobenzene in 22.3 ml of dichloromethane, the mixture was cooled on an ice bath and 2.87 ml (12.0 mmol) of di-tert-butyl dicarbonate was added thereto in an argon stream with stirring. After that, temperature of the mixture was raised up to room temperature and stirring was conducted for 1 hour. After the reaction was finished, the reaction solution was poured into 200 ml of water and the mixture was extracted with 200 ml of chloroform. The organic layer was successively washed with water, a saturated aqueous solution of sodium hydrogen carbonate and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate and concentrated in vacuo. The resulting powder was washed twice with each 10 ml of hexane to give 2.71 g of the title compound as white powder (yield: 90%). Rf value: 0.55 (n-hexane: ethyl acetate = 4:1 (v/v)) Mass spectrum (FAB, m/z): 300, 302 (M+ + 1) 1H-NMR spectrum (DMSO-d6, δ ppm): 1.27 (d, J = 6.8Hz, 3H), 1.36 (brs, 9H), 4.51-4.64 (m, 1H), 7.25 (d, J = 8.3Hz, 2H), 7.35-7.45 (m, 1H), 7.49 (d, J = 8.3Hz, 2H)
References:
Ube Industries, Ltd.;SANTEN PHARMACEUTICAL CO., LTD. EP1679308, 2006, A1 Location in patent:Page/Page column 32-33

24424-99-5
857 suppliers
$13.50/25G

90006-14-7
26 suppliers
$94.00/5g
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
49 suppliers
$45.00/50mg

110-05-4
276 suppliers
$22.00/100mL

68819-84-1
120 suppliers
$11.00/250mg
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
49 suppliers
$45.00/50mg