
BOC-4-AMINOBENZYLALCOHOL synthesis
- Product Name:BOC-4-AMINOBENZYLALCOHOL
- CAS Number:144072-29-7
- Molecular formula:C12H17NO3
- Molecular Weight:223.27

24424-99-5
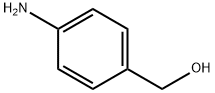
623-04-1

144072-29-7
General procedure: p-Aminobenzyl alcohol (1 g, 8.12 mmol, 1 eq.) was dissolved in 80 mL of anhydrous tetrahydrofuran (THF) under nitrogen protection. To this solution was sequentially added N,N-diisopropylethylamine (DIEA, 1.4 mL, 8.12 mmol, 1 eq.) and di-tert-butyl dicarbonate (Boc2O, 1.9 mL, 8.12 mmol, 1 eq.). The reaction mixture was heated to reflux and stirred overnight. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure in a rotary evaporator to remove the solvent. The residue was dissolved in ethyl acetate (EtOAc) and the organic layer was washed with 0.1 N hydrochloric acid solution to remove unreacted amine and DIEA.The organic layer was dried with anhydrous magnesium sulfate (MgSO4), filtered and concentrated again under reduced pressure. The crude product was purified by silica gel column chromatography with petroleum ether-ethyl acetate (1:1, v/v) as eluent to afford BOC-4-aminobenzyl alcohol (1.85 g, quantitative yield). The structure of the product was confirmed by 1H NMR and 13C NMR: 1H NMR (CDCl3) δ 1.49 (s, 9H), 2.17 (s, 1H), 4.53 (s, 2H), 6.83 (s, 1H), 7.19 (d, J = 8.5 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H); 13C NMR (CDCl3) δ 28.28, 64.54, 80.37, 118.49, 127.59, 135.31, 137.46, 152.72.

24424-99-5
867 suppliers
$13.50/25G

623-04-1
365 suppliers
$6.00/1g

144072-29-7
90 suppliers
$8.00/250mg
Yield: 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuranHeating / reflux;
Steps:
10
To a solution of p-amino-benzylalcohol (1 g, 8.12 mmol, 1 eq) in 80 mL of anhydrous THF were added DIEA (1.4 mL, 8.12 mmol, 1 eq) and BoC2O (1.9 mL, 8.12 mmol, 1 eq). The mixture was heated to reflux overnight, then cooled down and evaporated under vacuum. The residue was dissolved in EtOAc. The organic layer was washed with a 0.1 N HCl solution, dried over MgSO4, filtered and evaporated under vacuum. The crude product was purified by
References:
ENZON PHARMACEUTICALS, INC. WO2008/34124, 2008, A2 Location in patent:Page/Page column 72-73

144072-30-0
103 suppliers
$22.00/250mg

144072-29-7
90 suppliers
$8.00/250mg

34619-03-9
29 suppliers
inquiry

623-04-1
365 suppliers
$6.00/1g

144072-29-7
90 suppliers
$8.00/250mg

110969-44-3
19 suppliers
$333.00/5g

144072-29-7
90 suppliers
$8.00/250mg

24424-99-5
867 suppliers
$13.50/25G

150-13-0
943 suppliers
$5.00/25g

144072-29-7
90 suppliers
$8.00/250mg