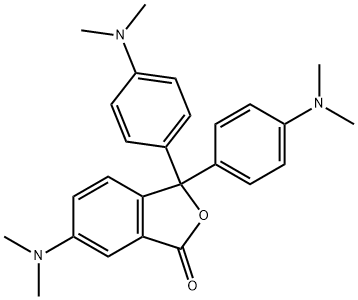
Crystal violet lactone synthesis
- Product Name:Crystal violet lactone
- CAS Number:1552-42-7
- Molecular formula:C26H29N3O2
- Molecular Weight:415.53
![2-[bis[4-(dimethylamino)phenyl]methyl]-5-(dimethylamino)benzoic acid](/CAS/GIF/1255-69-2.gif)
1255-69-2

1552-42-7
Example 1: 21.5 g (97.0% purity) of 2-[4,4'-bis(dimethylamino)diphenylmethyl]-5-dimethylaminobenzoic acid was added to a 500 mL three-necked flask, followed by 250 mL of deionized water. The resulting suspension was stirred with the addition of 21.5 g of activated carbon (Model: SHIRASAGI-C, manufactured by Takeda Chemical Industries) and 2.0 g of 49% NaOH aqueous solution. The reaction system was heated to 90-95°C and maintained in this temperature range. During the reaction, air was passed into the reaction system at a rate of 400 mL/min for 9 hours. Upon completion of the reaction, the reaction mixture was filtered while hot, the filter cake was collected and re-slurried with deionized water. 19.5 g of 36% HCl aqueous solution was slowly added to the slurried solution until the solid was completely dissolved. Subsequently, the activated carbon was removed by filtration. The pH of the filtrate was adjusted to 7.0 with 49% NaOH aqueous solution and crystals of the target product were precipitated. The crystals were collected by filtration, washed with cold water and dried under vacuum to give 20.1 g of 3,3-bis(4-dimethylaminophenyl)-6-dimethylaminophthalide in 95.4% yield. Analyzed by HPLC, the by-product generation rate was 1.5% and the reaction selectivity was 98.4%.
![2-[bis[4-(dimethylamino)phenyl]methyl]-5-(dimethylamino)benzoic acid](/CAS/GIF/1255-69-2.gif)
1255-69-2
2 suppliers
inquiry

1552-42-7
278 suppliers
$8.00/5g
Yield:1552-42-7 95.4%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in water
Steps:
1 EXAMPLE 1
EXAMPLE 1 To a 500 ml reaction vessel, 21.5 g of 2-[4,4'-bis(dimethylamino) benzhydryl]-5-dimethylaminobenzoic acid having a purity of 97.0% was charged and 250 g of water was added. To the dispersion thus obtained, 21.5 g of activated carbon; SHIRASAGI-C (manufactured by Takeda Chemical Ind. Co.) and 2.0 g of a 49% aqueous NaOH solution were added and stirred at 90 to 95° C. Through the reaction system thus obtained air was blown for 9 hours at a rate of 400 ml/min. After finishing the reaction, the reaction mass was filtered and the obtained cake was slurried in water, and completely dissolved by adding 19.5 g of a 36% aqueous HCl solution. Thereafter, the activated carbon was filtered. The filtrate was neutralized to pH7.0 with a 49% aqueous NaOH solution. The precipitated crystals were filtered, washed and dried to obtain 20.1 g of 3,3-bis(4-dimethylaminophenyl)-6-dimethylaminophthalide. The yield was 95.4%. Formation of by-product in the reaction was 1.5%. Selectivity of reaction was 98.4%.
References:
Mitsui Chemicals, Inc.;Yamamoto Chemicals US5973168, 1999, A
![3,3-bis[4-(dimethylamino)phenyl]phthalide](/CAS/GIF/5339-80-0.gif)
5339-80-0
2 suppliers
inquiry

1552-42-7
278 suppliers
$8.00/5g
![3,3-bis[4-(dimethylamino)phenyl]-6-nitro-2-benzofuran-1-one](/CAS/20180720/GIF/29199-08-4.gif)
29199-08-4
1 suppliers
inquiry

1552-42-7
278 suppliers
$8.00/5g

100-10-7
633 suppliers
$5.00/1g

1552-42-7
278 suppliers
$8.00/5g
