
Cyclohexanecarboxylic acid chloride synthesis
- Product Name:Cyclohexanecarboxylic acid chloride
- CAS Number:2719-27-9
- Molecular formula:C7H11ClO
- Molecular Weight:146.61

98-89-5

2719-27-9
The general procedure for the synthesis of cyclohexanecarboxylic acid from cyclohexanecarboxylic acid is as follows: 20.0 g (of which 6.95 g, 0.054 mol, of cyclohexanecarboxylic acid) of cyclohexanecarboxylic acid solution was added to 1.0 g of n-dodecane (as an internal standard) to a reactor fitted with a condenser and a Dean-Stark water separator. After adding 90 mL of benzene, about 25 mL of benzene was removed by distillation under nitrogen protection to prepare an anhydrous solution. After the reaction solution was cooled to room temperature, 6.0 mL (9.8 g, 0.082 mol) of thionyl chloride was added and the reaction was refluxed for 1 hour. After completion of the reaction, a portion of the reaction solution was taken and reacted with methanol containing a small amount of triethylamine, which was used to identify the product cyclohexanecarbonyl chloride. The yield of methyl cyclohexanecarboxylate was analyzed by gas chromatography (GC). The results showed that the conversion of cyclohexanecarboxylic acid chloride was more than 99% and the selectivity was higher than 99%.

98-89-5
395 suppliers
$13.00/25g

2719-27-9
357 suppliers
$16.00/25mL
Yield:2719-27-9 99%
Reaction Conditions:
with thionyl chloride in dodecane;benzene at 20; for 1 h;Heating / reflux;
Steps:
1.ii EXAMPLE 1
20.0 g (cyclohexanecarboxylic acid: 6.95 g, 0.054 mol) of the cyclohexanecarboxylic acid solution and 1.0 g of n-dodecane as an internal standard material were added to a reactor equipped with a condenser and a Dean-Stark water separator. After adding 90 ml of benzene, about 25 ml of benzene was distilled off in the nitrogen atmosphere to make the anhydrous solution. The temperature of the reactant solution was lowered to the atmospheric temperature and 6.0 ml (9.8 g, 0.082 mol) of thionyl chloride was added for one hour of reflux. [0046] After the reaction, part of the reactant solution was collected and reacted with methanol including a small amount of triethylamine in order to identify the product, cyclohexanecarbonyl chloride. The yield of methyl cyclohexane carboxylate was analyzed by GC. As a result, cyclohexanecarbonyl chloride was produced with a conversion rate of more than 99% and a selectivity of more than 99%.
References:
Korea Kumho Petrochemical Co., Ltd. US2004/73068, 2004, A1 Location in patent:Page 3

2043-61-0
340 suppliers
$6.00/5g

2719-27-9
357 suppliers
$16.00/25mL

707-74-4
22 suppliers
$284.00/1g

98-89-5
395 suppliers
$13.00/25g

2719-27-9
357 suppliers
$16.00/25mL

938-18-1
316 suppliers
$9.00/25g
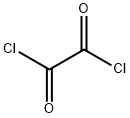
79-37-8
489 suppliers
$17.67/10gm:

110-82-7
771 suppliers
$10.00/25g

2719-27-9
357 suppliers
$16.00/25mL

65-85-0
1421 suppliers
$10.00/25 g

2719-27-9
357 suppliers
$16.00/25mL