
Cyclophosphamide synthesis
- Product Name:Cyclophosphamide
- CAS Number:50-18-0
- Molecular formula:C7H15Cl2N2O2P
- Molecular Weight:261.09

Yield:50-18-0 92.3%
Reaction Conditions:
with ammonia at 120; under 3040.2 Torr; for 2 h;Molecular sieve;
Steps:
1; 2; 3
(2) 10 g of 2-chloro-2-oxo-[1.3.2]-oxazaphosphinane was transferred to a pressure reaction bottle.Add 50g of dichloroethane and 10g of 5a molecular sieve (used after activation at 300 °C),Passing ammonia gas, maintaining at 4 atmospheres, heating to 120 ° C, reacting for 2 hours, the reaction is completed;(3) After filtering the reaction mixture, adding ice-water mixture, stirring for 30 minutes, separating the organic phase, washing with 10% hydrochloric acid solution, separating the organic phase, controlling the water bath temperature to 50 ° C, and concentrating the organic phase to dryness under reduced pressure. Acetone was added, the temperature was raised to 40 ° C to dissolve, and then the temperature was lowered to 5 ° C or less, and the crystal was decanted for 5 hours, and filtered to obtain a white crystalline solid, which was dried to obtain cyclophosphamide.
References:
CN109535201,2019,A Location in patent:Paragraph 0026; 0029; 0030; 0033-0037
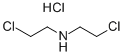
821-48-7
570 suppliers
$6.00/25g

156-87-6
482 suppliers
$5.00/10g

50-18-0
327 suppliers
$50.00/100mg
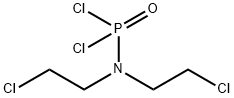
127-88-8
61 suppliers
$50.00/250mg

156-87-6
482 suppliers
$5.00/10g

50-18-0
327 suppliers
$50.00/100mg
![2H-1,3,2-Oxazaphosphorin-2-amine, tetrahydro-N,N-bis[2-[(trimethylsilyl)oxy]ethyl]-, 2-oxide](/CAS/20210305/GIF/195966-75-7.gif)
195966-75-7
0 suppliers
inquiry

50-18-0
327 suppliers
$50.00/100mg



