
ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate synthesis
- Product Name:ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate
- CAS Number:1489-97-0
- Molecular formula:C11H18O4
- Molecular Weight:214.26

17159-79-4

107-21-1
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
Ethyl 4-oxo-cyclohexanecarboxylate (52.8 g, 0.31 mol), ethylene glycol (67.4 g, 1.08 mol) and p-toluenesulfonic acid (0.7 g) were dissolved in toluene (160 mL) and the reaction mixture was stirred at room temperature. After the reaction was completed, the reaction solution was poured into ether (300 mL), washed sequentially with water, sodium bicarbonate solution and sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give a colorless liquid product. The product was directly used in the next reaction without further purification, yield: 66.5 g (100%).1H-NMR (CDCl3) δ: 1.24 (t, 3H), 1.53 (m, 2H), 1.76 (m, 4H), 1.92 (m, 2H), 2.31 (m, 1H), 3.91 (s, 4H), 4.11 (q, 2H).13C- NMR (CDCl3) δ: 14.28 (q), 26.32 (t), 33.76 (t), 41.59 (d), 60.14 (t), 64.21 (t), 107.90 (d), 174.77 (s). Sec-butanediol (1.08 mol) and p-toluenesulfonic acid (0.7 g) were added to a solution of ethyl 4-oxo-cyclohexanecarboxylate (0.31 mol) in toluene (160 mL) and stirred for 20 hr at 25 °C. At the end of the reaction, ethyl acetate (300 mL) was added, and the organic phase was separated, washed sequentially with water, saturated aqueous sodium bicarbonate solution, and sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure. The resulting product can be used in the next reaction without further purification.

17159-79-4
345 suppliers
$5.00/1g

107-21-1
1370 suppliers
$10.00/25g
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
139 suppliers
$17.00/1g
Yield:1489-97-0 100%
Reaction Conditions:
with toluene-4-sulfonic acid in toluene at 20 - 25; for 20 h;Product distribution / selectivity;
Steps:
a; A
4-Oxo-cyclohexanecarboxylic acid ethyl ester (52.8 g, 0.31 mol, Merck, order no. 814249, ethylene glycol (67.4 g, 1.08 mol) and p-toluenesulfonic acid (0.7 g) in toluene (160 ml) were stirred at RT for 20 h, the reaction solution was poured into diethyl ether (300 ml) and the mixture was washed with water, sodium bicarbonate solution and sodium chloride solution. The solution was dried (Na2SO4) and concentrated i. vac. and the colourless liquid which remained was processed further without purification.Yield: 66.5 g (100%)1H-NMR (CDCl3): 1.24 (t, 3H); 1.53 (m, 2H); 1.76 (m, 4H); 1.92 (m, 2H); 2.31 (m, 1H); 3.91 (s, 4H); 4.11 (q, 2H).13C-NMR (CDCl3): 14.28 (q); 26.32 (t); 33.76 (t); 41.59 (d); 60.14 (t); 64.21 (t); 107.90 (d); 174.77 (s).Stage AEthylene glycol (1.08 mol) and p-toluenesulfonic acid (0.7 g) were added to a solution of 4-oxo-cyclohexanecarboxylic acid ethyl ester (0.31 mol) in toluene (160 ml) and the mixture was stirred at 25° C. for 20 h. Ethyl acetate (300 ml) was then added. The organic phase separated off was washed with water, aqueous saturated NaHCO3 solution and NaCl solution. After drying of the organic phase over Na2SO4 and filtration, the solvent was removed in vacuo. The product was employed in the next stage without further purification.
References:
Gruenenthal GmbH US2008/306084, 2008, A1 Location in patent:Page/Page column 33-34; 44-45
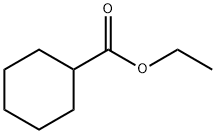
3289-28-9
111 suppliers
$13.00/1g

107-21-1
1370 suppliers
$10.00/25g
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
139 suppliers
$17.00/1g

17159-79-4
345 suppliers
$5.00/1g
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
139 suppliers
$17.00/1g

17159-79-4
345 suppliers
$5.00/1g
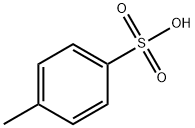
104-15-4
755 suppliers
$133.00/500g

107-21-1
1370 suppliers
$10.00/25g
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
139 suppliers
$17.00/1g
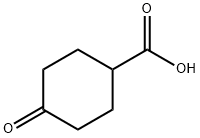
874-61-3
184 suppliers
$7.00/250mg
![ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate](/CAS/GIF/1489-97-0.gif)
1489-97-0
139 suppliers
$17.00/1g