
Ethyl 3-amino-4-pyrazolecarboxylate synthesis
- Product Name:Ethyl 3-amino-4-pyrazolecarboxylate
- CAS Number:6994-25-8
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15

94-05-3

1260243-04-6
General procedure for the synthesis of ethyl 5-amino-1H-pyrazole-4-carboxylate from ethyl ethoxymethylcyanoacetate: ethyl ethoxymethylcyanoacetate (68 g, 0.4 mol) was added to a reaction vessel containing 200 mL of anhydrous ethanol; 100 mL of hydrazine hydrate was added slowly and dropwise to the reaction vessel. The reaction mixture was placed in an electric heating jacket and gradually heated up to 80 °C within 30 min to obtain mixture A. The mixture A was heated to reflux and kept in reflux reaction for 4 h. The reaction was carried out under reduced pressure. Upon completion of the reaction, ethanol was removed by distillation under reduced pressure and the remaining solid was concentrated by evaporation. The concentrated solid was cooled to room temperature and left to precipitate a light yellow solid, which was filtered, washed and dried to give the intermediate ethyl 5-amino-1H-pyrazole-4-carboxylate. Cold anhydrous ethanol was used for the washing process. The mass of the final product ethyl 5-amino-1H-pyrazole-4-carboxylate was 41.68 g and the yield was 66.78%.
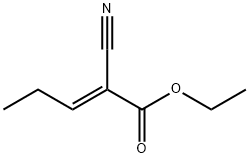
144918-37-6
1 suppliers
inquiry

6994-25-8
414 suppliers
$5.00/1g
Yield:6994-25-8 99%
Reaction Conditions:
with hydrazine in ethanol at 20;Reflux;
Steps:
To a stirred solution of ethyl (E)-2-cyano-3-ethoxyacrylate (5 g, 29.5 mmol) in ethanol (50 mL) wasadded hydrazine (1.8 mL, 29.5 mmol) dropwise. The mixture was stirred at room temperature for10 mins and was heated to reflux overnight. The solvent was removed in vacuo and the residue wasextracted with ethyl acetate, washed with H2O and brine. The water layer was extracted withethyl acetate for three times. The organic phase was dried over anhydrous sodium sulfate,filtered, and concentrated to afford compound 42a as light-yellow solid (4.54 g, 99%).
References:
Chen, Yun;Bai, Gang;Ning, Yi;Cai, Shi;Zhang, Tao;Song, Peiran;Zhou, Jinpei;Duan, Wenhu;Ding, Jian;Xie, Hua;Zhang, Huibin [European Journal of Medicinal Chemistry,2020,vol. 190,art. no. 112092] Location in patent:supporting information

94-05-3
287 suppliers
$5.00/5g

6994-25-8
414 suppliers
$5.00/1g

6630-64-4
23 suppliers
inquiry

6994-25-8
414 suppliers
$5.00/1g

42466-67-1
37 suppliers
inquiry

6994-25-8
414 suppliers
$5.00/1g

94-05-3
287 suppliers
$5.00/5g

6994-25-8
414 suppliers
$5.00/1g