
Ethyl iMidazo[1,5-a]pyridine-8-carboxylate synthesis
- Product Name:Ethyl iMidazo[1,5-a]pyridine-8-carboxylate
- CAS Number:697739-12-1
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2

64-18-6
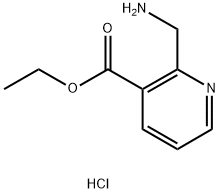
697739-11-0

108-24-7
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
Acetic anhydride (38.21 mL, 405 mmol) and formic acid (15.28 mL, 405 mmol) were mixed and reacted at 60 °C for 2 h and subsequently cooled to room temperature. To this reaction mixture was added ethyl 2-(aminomethyl)-3-pyridinecarboxylate hydrochloride (17.55 g, 81 mmol), and the resulting mixture was stirred at room temperature for 1 hour and then heated at 35°C for 3 hours. After completion of the reaction, the mixture was cooled to 5 °C and neutralized with 0.88 aqueous ammonia solution, followed by extraction with dichloromethane (3×). The combined dichloromethane layers were washed sequentially with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography to afford ethyl imidazo[1,5-a]pyridine-8-carboxylate (8.67 g, 56% yield) using dichloromethane solution containing 2% methanol (containing 0.5% ammonia) as eluent.

64-18-6
1126 suppliers
$22.12/250ML

697739-11-0
17 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
33 suppliers
$149.00/100mg
Yield:697739-12-1 56%
Reaction Conditions:
Stage #1: formic acid;acetic anhydride at 60; for 2 h;
Stage #2: ethyl 2-(aminomethyl)-3-pyridinecarboxylate hydrochloride at 20 - 35; for 4 h;
Steps:
36 Description 36: Ethyl IMIDAZOFL, 5-ALPYRIDINE-8-CARBOXYLATE
Acetic anhydride (38. 21 ml, 405 mmol) and formic acid (15.28 ml, 405 mmol) were mixed together at 60 °C for 2 hours then allowed to cool to room temperature. To this mixture was added Description 35 (17.55 g, 81 mmol), and the resulting mixture stirred at room temperature for 1 hour, then heated at 35 °C for 3 hours. The mixture was cooled to 5 °C and neutralised with 0.88 ammonia solution and then extracted with dichloromethane (3 x). The combined dichloromethane layers were washed with water, saturated NACI, dried over NA2S04, filtered and evaporated. The residue was purified by column chromatography on silica eluting with 2% MEOH in DCM + 0.5% NH40H to give the title compound (8.67 g, 56%).
References:
WO2004/46133,2004,A1 Location in patent:Page 32

64-18-6
1126 suppliers
$22.12/250ML

697739-11-0
17 suppliers
inquiry
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
33 suppliers
$149.00/100mg

697739-11-0
17 suppliers
inquiry
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
33 suppliers
$149.00/100mg
614-18-6
478 suppliers
$5.00/10g
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
33 suppliers
$149.00/100mg

14906-63-9
15 suppliers
inquiry
![Ethyl iMidazo[1,5-a]pyridine-8-carboxylate](/CAS/20150408/GIF/697739-12-1.gif)
697739-12-1
33 suppliers
$149.00/100mg