
eupatilin synthesis
- Product Name:eupatilin
- CAS Number:22368-21-4
- Molecular formula:C18H16O7
- Molecular Weight:344.32
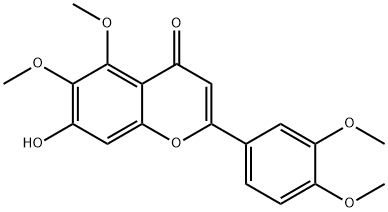
40983-99-1

22368-21-4
The general procedure for the synthesis of 2-(3,4-dimethoxyphenyl)-5,7-dihydroxy-6-methoxy-4H-benzopyran-4-one from 5,7-dihydroxy-3',4',6-trimethoxyflavone was as follows: 7-hydroxy-3',4',5,6-tetramethoxyflavone (4.44 g, 12.4 mmol) was suspended in 88 mL of acetonitrile. Aluminum trichloride (8.27 g, 5 eq.) was added to the suspension at room temperature and the reaction mixture was subsequently heated to reflux for 1.5 hours. After completion of the reaction, the solvent was removed by evaporation under reduced pressure. To the residue, 10% aqueous hydrochloric acid and chloroform were added and the mixed solution was heated to reflux until it became clear. After the solution was cooled to room temperature, the organic layer was separated, washed sequentially with water and brine, and then dried with anhydrous magnesium sulfate. The solvent of the organic layer was removed by evaporation under reduced pressure to obtain the crude product. The crude product was recrystallized in methanol to give 3.18 g of the target compound in 74% yield. The structure of the product was confirmed by NMR (CDCl3): δ 13.05 (s, 1H), 7.50 (dd, J = 8.6, 2.2 Hz, 1H), 7.31 (d, J = 2.1 Hz, 1H), 6.96 (d, J = 8.5 Hz, 1H), 6.59 (s, 1H), 6.56 (s, 1H), 6.48 (br s, 1H). 4.03 (s, 3H), 3.96 (s, 3H), 3.95 (s, 1H).

40983-99-1
0 suppliers
inquiry

22368-21-4
217 suppliers
$27.00/1mg
Yield:22368-21-4 74%
Reaction Conditions:
with hydrogenchloride;aluminium trichloride in chloroform;acetonitrile
Steps:
2 5,7-dihydroxy-3',4',6-trimethoxy flavone
EXAMPLE 2 5,7-dihydroxy-3',4',6-trimethoxy flavone After 7-hydroxy-3',4',5,6-tetramethoxy flavone (4.44 g, 12.4 mmol) was suspended in 88 mL of acetonitrile and aluminum trichloride (8.27 g, 5 equivalents) was added hereto at room temperature, the reaction mixture was refluxed for 1.5 hour and the solvent was removed by evaporation under reduced pressure. To the residue was added 10% aqueous solution of hydrochloric acid and chloroform, then the solution was refluxed until it became clear. After the solution was cooled to room temperature, the organic layer was washed with water and brine, then dried over anhydrous magnesium sulfate and the solvent of the organic layer was removed by reduced pressure. The residue was recrystallized in methanol to afford 3.18 g of the product (74%). NMR(CDCl3): 13.05(s,1H), 7.50(dd, J=8.6, 2.2 Hz, 1H), 7.31(d, J=2.1 Hz, 1H), 6.96(d, J=8.5 Hz, 1H), 6.59(s, 1H), 6.56(s, 1H), 6.48(br s, 1H), 4.03(s, 3H), 3.96(s, 3H), 3.95(s, 1H).
References:
Dong a Pharmaceutical Co., Ltd. US6025387, 2000, A

480-40-0
560 suppliers
$11.00/5g

22368-21-4
217 suppliers
$27.00/1mg

110506-85-9
17 suppliers
inquiry

22368-21-4
217 suppliers
$27.00/1mg

681294-57-5
11 suppliers
inquiry

22368-21-4
217 suppliers
$27.00/1mg

39548-89-5
32 suppliers
inquiry

22368-21-4
217 suppliers
$27.00/1mg