
FMOC-L-2-FURYLALANINE synthesis
- Product Name:FMOC-L-2-FURYLALANINE
- CAS Number:159611-02-6
- Molecular formula:C22H19NO5
- Molecular Weight:377.39
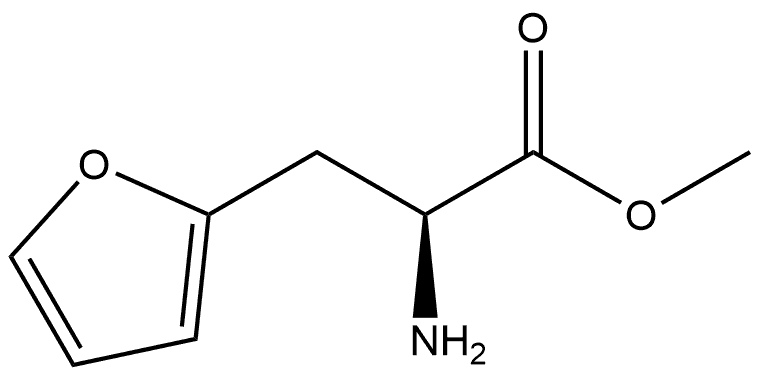
146725-85-1

28920-43-6

159611-02-6
Methyl (S)-2-amino-3-(furan-2-yl)propanoate (1 g, 6.5 mmol) was dissolved in toluene as the starting material. 2M LiOH aqueous solution (3.27 ml, 6.5 mmol) and dioxane (3.27 ml) were slowly added to the reaction system at 0 °C under nitrogen protection and the reaction mixture was stirred at room temperature overnight. The progress of the reaction was monitored by TLC (Spreader: CHCl3/MeOH/NH3 aqueous solution = 1/1/0.1) to confirm complete hydrolysis of the ester group. Subsequently, 1M aqueous NaHCO3 (9.75 ml, 9.75 mmol) dissolved in dioxane (10 ml) and methyl 9-fluorenyl chloroformate (2.5 g, 9.75 mmol) were added to the reaction solution and stirring was continued for 1 hour. After completion of the reaction, the dioxane was removed by distillation under reduced pressure and the remaining aqueous phase was acidified with 10% KHSO4 solution to pH=2. The aqueous phase was extracted with CHCl3 (3×20 ml), the organic phases were combined and dried over anhydrous MgSO4. After concentration under reduced pressure, the target product (S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-(furan-2-yl)propanoic acid (1.3 g, 53% yield) was purified by fast column chromatography (eluent: hexane/ethyl acetate/acetic acid=10/10/1) as white crystals. The product was characterized by 1H NMR and 13C NMR and the data were consistent with the expected structure.

146725-85-1
15 suppliers
inquiry

28920-43-6
546 suppliers
$5.00/5g

159611-02-6
127 suppliers
$22.00/100mg
Yield: 1.3 g (53%)
Reaction Conditions:
with LiOH;sodium hydrogencarbonate in 1,4-dioxane
Steps:
6.c c
c (S)-2-(9-Fluorenylmethyloxycarbonylamino)-3-(2-furyl)-propionic acid Methyl (S)-2-amino-3-(2-furyl)propionate (1 g, 6.5 mmol) was mixed with 2M LiOH (3.27 ml, 6.5 mmol) and dioxane (3.27 ml) at 0° C. and stirred overnight under N2. The next day a TLC control (CHCl3 /MeOH/NH3 aq 1/1/0.1) of the reaction mixture showed no ester present. 1M NaHCO3 (9.75 ml, 9.75 mmol) and 9-fluorenylmethyloxycarbonyl chloride (2.5 g, 9.75 mmol) dissolved in dioxane (10 ml) were added to the above solution and the stirring continued for a further 1 hour. Dioxane was removed under reduced pressure and the aqueous solution acidified with a 10% KHSO4 solution to pH 2. The solution was extracted with CHCl3 (3*20 ml), dried (MgSO4), evaporated and purified by flash chromatography (Hexane/ethyl acetate/acetic acid 10/10/1). Yield: 1.3 g (53%), white crystals. 1 H NMR (CDCl3): δ2.95 (dd, 1H, J 9.9, J 15.2 Hz), 3.08 (dd, 1H, J 4.4, J 15.2 Hz), 3.6 (br.s, 1H), 4.16-4.26 (m, 4H), 6.12 (d, 1H, J 2.9 Hz), 6.33 (t, 1H, J 2.3 Hz), 7.29 (t, 2H, J 7.32 Hz), 7.39 (t, 2H, J 7.48 Hz), 7.49 (br.s, 1H), 7.64 (dd, 2H, J 7.17, J 2.14 Hz), 7.85 (d, 2H, J 7.48 Hz). 13 C NMR (CDCl3): δ29.8, 46.98, 66.04, 107.45, 110.85, 120.48, 125.57, 125.63, 127.50, 128.07, 141.06, 142.21, 144.09, 151.84, 156.26, 160.35.
References:
Nycomed Imaging AS US5629293, 1997, A