
Glycine, N-(phenylMethyl)-, Methyl ester synthesis
- Product Name:Glycine, N-(phenylMethyl)-, Methyl ester
- CAS Number:53386-64-4
- Molecular formula:C10H13NO2
- Molecular Weight:179.22

96-32-2
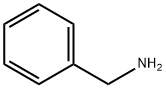
100-46-9

53386-64-4
The general procedure for the synthesis of methyl 2-(benzylamino)acetate from methyl bromoacetate and benzylamine was as follows: the flame-dried flask was cooled under nitrogen protection and a tetrahydrofuran (THF, 10 mL, 1 M) solution of benzylamine (2.36 mL, 21.6 mmol) was added. The reaction system was cooled to 0 °C, followed by slow dropwise addition of methyl bromoacetate (0.93 mL, 9.8 mmol). The reaction mixture was controlled to gradually warm up to room temperature over 2.5 h and the progress of the reaction was monitored by thin layer chromatography (TLC) until the feedstock was completely consumed. After completion of the reaction, the mixture was concentrated under reduced pressure and the resulting residue was dissolved in ether (Et2O) and filtered. The filtrate was concentrated again under reduced pressure and the crude product was purified by fast column chromatography to give 1.12 g (63% yield) of methyl 2-(benzylamino)acetate as a colorless oil. Its nuclear magnetic resonance hydrogen spectrum (1H NMR, 500 MHz, CDCl3) data were as follows: δ 7.30 (d, J = 4.3 Hz, 4H), 7.29-7.24 (m, 1H), 3.81 (s, 2H), 3.73 (s, 3H), 3.43 (s, 2H), 1.93 (s, br, 1H), which is in accordance with the literature reports.

67-56-1
782 suppliers
$7.29/5ml-f
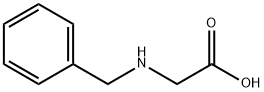
17136-36-6
232 suppliers
$10.00/1g

53386-64-4
43 suppliers
$10.00/250mg
Yield: 100%
Reaction Conditions:
with thionyl chloride in methanol at 0; for 18 h;
Steps:
51
To a stirred solution of N-benzylglycine (820 mg, 4 mmol) and methanol (8 mL) was added thionyle chloride (2 mL) dropwise at 0 °C. The mixture was stirred for 18 h. Then the solvent was evaporated under reduced pressure to give the compound benzylamino-acetic acid methyl ester as a white solid. Yield 100%. 1H NMR (DMSO-d6) δ ppm: 7.55-7.58 (m, 2H), 7.41-7.43 (m, 3H), 4.16 (s, 2H), 3.99 (s, 2H), 1.72 (s, 3H). tR,LCMS = 2.01 min; Purity 99%; MS (ESI+): m/z = 180 (M + H)+.
References:
Charton, Julie;Gauriot, Marion;Totobenazara, Jane;Hennuyer, Nathalie;Dumont, Julie;Bosc, Damien;Marechal, Xavier;Elbakali, Jamal;Herledan, Adrien;Wen, Xiaoan;Ronco, Cyril;Gras-Masse, Helene;Heninot, Antoine;Pottiez, Virginie;Landry, Valerie;Staels, Bart;Liang, Wenguang G.;Leroux, Florence;Tang, Wei-Jen;Deprez, Benoit;Deprez-Poulain, Rebecca [European Journal of Medicinal Chemistry,2015,vol. 90,p. 547 - 567]

66646-88-6
24 suppliers
inquiry

53386-64-4
43 suppliers
$10.00/250mg

96-32-2
348 suppliers
$15.00/5g

100-46-9
504 suppliers
$5.00/5 g

53386-64-4
43 suppliers
$10.00/250mg

96-34-4
367 suppliers
$10.00/25g

100-46-9
504 suppliers
$5.00/5 g

53386-64-4
43 suppliers
$10.00/250mg
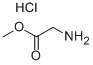
5680-79-5
541 suppliers
$3.66/25g
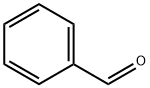
100-52-7
976 suppliers
$17.30/2g

53386-64-4
43 suppliers
$10.00/250mg