IMD-0354 synthesis
- Product Name:IMD-0354
- CAS Number:978-62-1
- Molecular formula:C15H8ClF6NO2
- Molecular Weight:383.67
321-14-2

328-74-5

978-62-1
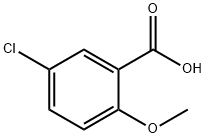
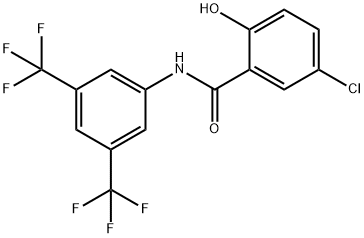
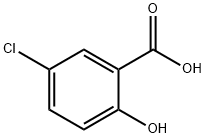
Using 5-chlorosalicylic acid (6.90 g, 40 mmol) and m-bis(trifluoromethyl)aniline (9.16 g, 40 mmol) as raw materials, phosphorus trichloride (1.74 mL, 20 mmol) and toluene (80 mL) were added and the reaction was carried out at reflux for 3 hours under argon protection. After completion of the reaction, the mixture was cooled to room temperature and diluted with ethyl acetate (240 mL). The organic layer was washed sequentially with water and saturated saline, and then dried with anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate=4:1, v/v) to afford N-[3,5-bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide (13.12 g, 85.5% yield) as a light yellow solid.1H-NMR (DMSO-d6) δ: 7.05 (1H, d, J= 8.7 Hz), 7.49 (1H, dd, J=8.7, 2.7 Hz), 7.85 (1H, s), 7.87 (1H, d, J=2.7 Hz), 8.45 (2H, s), 10.85 (1H, s), 11.39 (1H, s).

321-14-2
391 suppliers
$15.00/25g

328-74-5
369 suppliers
$10.00/1g

978-62-1
110 suppliers
$29.00/1mg
Yield: 85.5%
Steps:
51 Example 51
Example 51 N-[3,5-Bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide (Comopund No. 50). Using 5-chlorosalicylic acid and 3,5-bis(trifluoromethyl)aniline as the raw materials, the same operation as the example 16 gave the title compound. Yield: 85.5%. 1H-NMR(DMSO-d6): δ 7.05(1H, d, J=8.7Hz), 7.49(1H, dd, J=8.7, 2.7Hz), 7.85(1H, s), 7.87(1H, d, J=2.7Hz), 8.45(2H, s), 10.85(1H, s), 11.39(1H, s).
References:
Institute of Medicinal Molecular Design, Inc. EP1352650, 2003, A1

321-14-2
391 suppliers
$15.00/25g

978-62-1
110 suppliers
$29.00/1mg