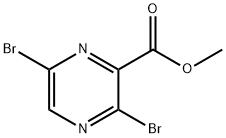
methyl 3,6-dibromopyrazine-2-carboxylate synthesis
- Product Name:methyl 3,6-dibromopyrazine-2-carboxylate
- CAS Number:13301-04-7
- Molecular formula:C6H4Br2N2O2
- Molecular Weight:295.92

6966-01-4

13301-04-7
Step 2: Methyl 3-amino-6-bromopyrazine-2-carboxylate (28.5 g, 120 mmol) was suspended in a mixed aqueous solution of 48% hydrobromic acid (120 mL) and acetic acid (30 mL) and cooled to 0 °C. The solution was then slowly added dropwise over a period of 45 minutes. Subsequently, a solution of bromine (18 mL, 336 mmol) in acetic acid (18 mL) was added slowly and dropwise over 45 minutes. While maintaining the reaction temperature at 0 °C, a solution of sodium nitrite (8.28 g, 420 mmol) in water (15 mL) was added and stirring was continued for 30 min. The reaction progress was monitored by thin layer chromatography (TLC). Upon completion of the reaction, excess bromine was quenched by dropwise addition of 30% aqueous sodium bisulfite solution (180 mL). The resulting precipitate was collected by filtration and purified by silica gel column chromatography using a hexane solution of 10% ethyl acetate as eluent to afford methyl 3,6-dibromopyrazine-2-carboxylate as a white crystalline solid (18 g, 49% yield). Mass spectrum (electrospray ionization) m/z: 294 ([M+H]+).

6966-01-4
223 suppliers
$8.00/1g

13301-04-7
148 suppliers
$7.00/250mg
Yield:13301-04-7 49%
Reaction Conditions:
Stage #1:methyl 3-amino-6-bromopyrazine-2-carboxylate with hydrogen bromide;bromine;acetic acid in water at 0; for 0.75 h;
Stage #2: with sodium nitrite in water at 0; for 0.5 h;Product distribution / selectivity;
Steps:
58.2
Step 2; A suspension of compound 2 (28.5 g, 120 mmol) in an aqueous solution of 48% HBr (120 mL) and acetic acid (30 mL) was cooled to 0° C., and then treated with a solution of bromine (18 mL, 336 mmol) in acetic acid (18 mL) over a period of 45 min. A solution of NaNO2 (8.28 g, 420 mmol) in water (15 ml) was added while maintaining the temperature at 0° C., stirring was continued for further 30 min. After completion of reaction (reaction progress was monitored by TLC), the excess bromine was quenched by the drop wise addition of 30% aqueous solution of NaHSO3 (180 mL). The resulting precipitate was filtered and purified by column chromatography on silica gel eluted with 10% ethyl acetate in hexane to afford compound 3 as a white crystalline solid (18 g, 49%). MS m/z (ES): 294 (M+H)+.
References:
de Vicente Fidalgo, Javier;Hermann, Johannes Cornelius;Lemoine, Remy;Li, Hongju;Lovey, Allen John;Sjogren, Eric Brian;Soth, Michael US2011/59118, 2011, A1 Location in patent:Page/Page column 84-85
16298-03-6
336 suppliers
$7.00/1g

13301-04-7
148 suppliers
$7.00/250mg

5424-01-1
352 suppliers
$10.00/5g

13301-04-7
148 suppliers
$7.00/250mg