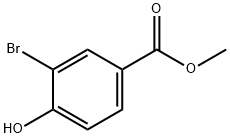
Methyl 3-bromo-4-hydroxybenzoate synthesis
- Product Name:Methyl 3-bromo-4-hydroxybenzoate
- CAS Number:29415-97-2
- Molecular formula:C8H7BrO3
- Molecular Weight:231.04

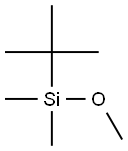
67-56-1

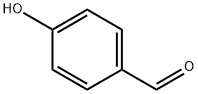
14348-41-5

29415-97-2
The general procedure for the synthesis of methyl 3-bromo-4-hydroxybenzoate from methanol and 3-bromo-4-hydroxybenzoic acid is as follows: 1. Preparation of intermediate T3.1 (methyl 3-bromo-4-hydroxybenzoate): - Under stirring conditions, 3-bromo-4-hydroxybenzoic acid (50.0 g, 231 mmol) was dissolved in methanol (300 mL). - To the above solution was slowly added cold sulfuric acid solution (2.50 mL, 47 mmol). - The reaction mixture was heated to 80 °C and the progress of the reaction was monitored by thin layer chromatography (TLC). - After 16.5 h of reaction, the solvent was removed by distillation under reduced pressure. - The reaction mixture was diluted with ethyl acetate (EtOAc). - The organic phase was sequentially washed twice with saturated aqueous sodium bicarbonate (NaHCO3), once with brine and then dried with anhydrous sodium sulfate. - After filtration, the organic solvent was removed by distillation under reduced pressure to give the intermediate T3.1 as a white solid in 100% yield, which can be used in the next reaction without further purification.

67-56-1
782 suppliers
$7.29/5ml-f

14348-41-5
173 suppliers
$8.00/1g

29415-97-2
174 suppliers
$5.00/1g
Yield:29415-97-2 100%
Reaction Conditions:
with sulfuric acid at 80;Cold solution;
Steps:
Intermediate T3T3.1[0304] Methyl 3-bromo-4-hydroxybenzoate (T3.1). To a stirred solution of 3- bromo-4-hydroxybenzoic acid (available from Alfa Aesar, Avocado, Lancaster) (50.0 g, 231 mmol) in MeOH (300 mL) was added a cold solution of sulfuric acid (2.50 mL, 47 mmol). The mixture was heated to 80°C and monitored by TLC. After 16.5 hours, the solvent was removed and the reaction mixture was diluted with EtOAc. The organic phase was washed carefully two times with saturated aqueous NaHCO3, once with brine, and then dried over anhydrous sodium sulfate. After filtration, the organic solvent was removed in vacuo to yield T3.1 as a white solid (yield 100%) that was used without purification.
References:
AMGEN INC.;BROWN, Sean P.;DRANSFIELD, Paul;DU, Xiaohui;FU, Zice;HOUZE, Jonathan;JIAO, XianYun;LAI, SuJen;LI, An-Rong;LIU, Jiwen;MA, Zhihua;MEDINA, Julio C.;PATTAROPONG, Vatee;SHEN, Wang;VIMOLRATANA, Marc;WANG, Yingcai;WANG, Zhongyu;YU, Ming;ZHU, Liusheng WO2010/45258, 2010, A2 Location in patent:Page/Page column 119
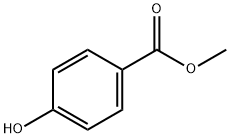
99-76-3
789 suppliers
$5.00/25g

29415-97-2
174 suppliers
$5.00/1g

14348-41-5
173 suppliers
$8.00/1g

18107-18-1
210 suppliers
$33.00/5g

29415-97-2
174 suppliers
$5.00/1g

99-76-3
789 suppliers
$5.00/25g

41727-47-3
112 suppliers
$23.19/10g

29415-97-2
174 suppliers
$5.00/1g