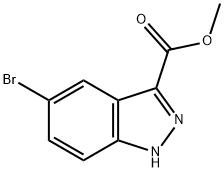
METHYL 5-BROMO-1H-INDAZOLE-3-CARBOXYLATE synthesis
- Product Name:METHYL 5-BROMO-1H-INDAZOLE-3-CARBOXYLATE
- CAS Number:78155-74-5
- Molecular formula:C9H7BrN2O2
- Molecular Weight:255.07

1077-94-7

67-56-1

78155-74-5
Step 2: Concentrated sulfuric acid (1 mL) was slowly added to a suspension of 5-bromo-1H-indazole-3-carboxylic acid (1.30 g, 5.39 mmol) in anhydrous methanol (50 mL) under argon protection. The reaction mixture was heated to reflux for 4 hours. Upon completion of the reaction, the solution was cooled to room temperature and subsequently evaporated under reduced pressure to remove methanol. The residue was dissolved in ethyl acetate and washed with deionized water. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to afford methyl 5-bromo-1H-indazole-3-carboxylate (1.35 g, 5.29 mmol, 98% yield) as a white solid. The product was confirmed by 1H NMR (DMSO-d6): δ 14.13 (s, 1H), 8.21 (d, J = 1.6 Hz, 1H), 7.67 (d, J = 7.2 Hz, 1H), 7.59 (dd, J = 7.2, 1.2 Hz, 1H), 3.92 (s, 3H); ESIMS analysis result: C9H7BrN2O2 m/z 256.0 (M + H).

1077-94-7
186 suppliers
$11.00/250mg

67-56-1
782 suppliers
$9.00/25ml

78155-74-5
116 suppliers
$9.00/100mg
Yield:78155-74-5 98%
Reaction Conditions:
with sulfuric acid for 4 h;Reflux;Inert atmosphere;
Steps:
25.2 Step 2
Concentrated sulfuric acid (1 mL) was added to a suspension of 5- bromo-lH-indazole-3-carboxylic acid (CXV) (1.30 g, 5.39 mmol) in dry MeOH (50 mL) and heated to reflux for 4 h under argon. The solution was cooled to room temperature and the MeOH was evaporated under vacuum. The residue was dissolved in EtOAc and washed with water. The organic phase was dried over Na2S04, filtered and concentrated to afford methyl 5-bromo-lH-indazole-3-carboxylate (CXVI) as a white solid (1.35 g, 5.29 mmol, 98% yield). 1H NMR (DMSO-d6) δ ppm 14.13 (s, 1H), 8.21 (d, J = 1.6 Hz, 1H), 7.67 (d, J= 7.2 Hz, 1H), 7.59 (dd, J= 7.2, 1.2 Hz, 1H), 3.92 (s, 3H); ESIMS found for C9H7BrN202 mlz 256.0 (M+H).
References:
WO2013/40215,2013,A1 Location in patent:Paragraph 00340

43120-28-1
173 suppliers
$10.00/1g

78155-74-5
116 suppliers
$9.00/100mg

67-56-1
782 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

459133-66-5
158 suppliers
$10.00/250mg

78155-74-5
116 suppliers
$9.00/100mg

4498-67-3
493 suppliers
$6.00/5g

78155-74-5
116 suppliers
$9.00/100mg

87-48-9
293 suppliers
$10.00/5g

78155-74-5
116 suppliers
$9.00/100mg