
MonoMethylauristatin F synthesis
- Product Name:MonoMethylauristatin F
- CAS Number:745017-94-1
- Molecular formula:C39H65N5O8
- Molecular Weight:731.96
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(αR,βR,2S)-β-methoxy-α-methyl-2-pyrrolidinepropanoyl-](/CAS/20211123/GIF/1369427-27-9.gif)
1369427-27-9

745017-94-1
The synthesis of (S)-2-((2R,3R)-3-((S)-1-((3R,4S,5S)-4-((S)-N,3-dimethyl-2-((S)-3-methyl-2-(methylaminopropionamido)butanamido)butanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl) was carried out using the compound (CAS: 1369427-27-9) as starting material. (methylamino)butanamido)butanamido)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid was prepared in the following general steps: (S)-2-((2R,3R)-3-((S)-1-((6S,9S,12S,13R)-12-((S)-sec-butyl)-6,9-diisopropyl-13-methoxy-2,2,5,11-tetramethyl-4,7,10-trioxo-3- oxa-5,8,11-triazatetradecan-15-yl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid (25 mg, 0.030 mmol) was dissolved in a mixture of 0.3 ml of HCl and 0.9 ml of 1,4-dioxane and stirred at room temperature for 35 min. The reaction mixture was diluted with 1.0 ml of ethanol and 1.0 ml of toluene, subsequently concentrated and evaporated again with ethanol/toluene (2:1) to afford the target product (S)-2-((2R,3R)-3-((S)-1-((3R,4S,5S)-4-((S)-N,3-dimethyl-2-((S)-3-methyl-2-(methylamino)butyramido)butanamide (S)-3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropanamido)-3-phenylpropanoic acid as a white solid (22 mg, about 400% yield), which can be used in the next reaction without further purification.LC-MS (ESI) m/z calculated value: C39H66N5O8 [M + H]+: 732.48, measured value: 732.60.
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(αR,βR,2S)-β-methoxy-α-methyl-2-pyrrolidinepropanoyl-](/CAS/20211123/GIF/1369427-27-9.gif)
1369427-27-9
1 suppliers
inquiry

745017-94-1
195 suppliers
$17.00/1mg
Yield:745017-94-1 100%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;water at 20; for 0.583333 h;
Steps:
40 Example 40. (S)-2-((2R,3R)-3-((S)- 1 -((3R,4S ,5S)-4-((S)-N,3-dimethyl-2-((S)-3- methyl-2-(methylamino)butanamido)butanamido)-3 -methoxy-5-methylheptanoyl)-pyrrolidin-2-yl)-3 -methoxy-2-methylpropanamido)-3 -phenylpropanoic acid (101)
(S)-2-((2R,3R)-3-((S)- 1 -((6S ,9S, 12S, 1 3R)- 12-((S)-sec-butyl)-6,9-diisopropyl- 13- methoxy-2,2,5,11 -tetramethyl-4,7, 1 0-trioxo-3 -oxa-5 ,8,11 -triazapenta-decan- 15- oyl)pyrrolidin-2-yl)-3 -methoxy-2-methylpropanamido)-3 -phenylpropanoic acid (25 mg,0.030 mmol) in the mixture of HC1 (conc. 0.3 ml) and 1,4-dioxane (0.9 ml) was stirred at RT for 35 mm. The mixture was diluted with EtOH (1.0 ml) and toluene (1.0 ml), concentrated and re-evaporated with EtOH/toluene (2:1) to afford the title compound as a white solid (22 mg, -400% yield) for the next step without further purification. LC-MS (ESI) mlz calcd. for C39H66N508 [M+Hj: 732.48, found: 732.60.
References:
WO2015/155753,2015,A2 Location in patent:Page/Page column 101
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(αR,βR,2S)-β-methoxy-α-methyl-2-pyrrolidinepropanoyl-, methyl ester](/CAS/20211123/GIF/1415246-61-5.gif)
1415246-61-5
1 suppliers
inquiry

745017-94-1
195 suppliers
$17.00/1mg
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(αR,βR,2S)-β-methoxy-α-methyl-2-pyrrolidinepropanoyl-, methyl ester](/CAS/20211123/GIF/1415246-55-7.gif)
1415246-55-7
2 suppliers
inquiry

745017-94-1
195 suppliers
$17.00/1mg

13734-41-3
563 suppliers
$8.00/1g

745017-94-1
195 suppliers
$17.00/1mg
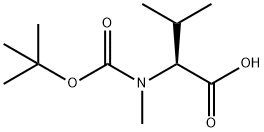
45170-31-8
251 suppliers
$29.00/1g

745017-94-1
195 suppliers
$17.00/1mg