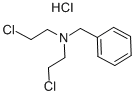
N-BENZYL-BIS(2-CHLOROETHYL)AMINE HYDROCHLORIDE synthesis
- Product Name:N-BENZYL-BIS(2-CHLOROETHYL)AMINE HYDROCHLORIDE
- CAS Number:10429-82-0
- Molecular formula:C11H16Cl3N
- Molecular Weight:268.61

101-32-6

10429-82-0
General procedure for the synthesis of N-benzyl-bis(2-chloroethyl)amine hydrochloride from 2,2'-(benzylimino)diethanol: A solution of sulfur dichloride (35.8 mL, 482 mmol) in anhydrous dichloromethane (60 mL) was added slowly and dropwise to a dichloromethane (200 mL) solution of 2,2'-(benzylimino)diethanol (obtained in step a, 31.4 g, 160 mmol). mL) in a cold solution of methylene chloride (200 mL). The reaction mixture was stirred overnight at room temperature. Upon completion of the reaction, the solvent was removed to dryness by rotary evaporation to afford N-benzyl-bis(2-chloroethyl)amine hydrochloride (47.2 g, 100% yield), and the product was used directly in the next step without further purification.HPLC-MS (Method A): retention time, 2.34 min; ESI-MS m/z, 262 (M + 1).

101-32-6
149 suppliers
$31.00/1g

10429-82-0
159 suppliers
$7.00/1g
Yield:10429-82-0 100%
Reaction Conditions:
with thionyl chloride in dichloromethane at 20;
Steps:
9A.b b) N-Benzyl-2-chloro-N-(2-chloroethyl)ethanam me hydrochloride.
Sulfurous dichloride (35.8mL, 482 mmol) in anhydride DCM (60 mL), was added to a cold solution of 2,2’-(benzylazanediyl)diethanol (obtained in step a, 31.4 g, 160 mmol) in DCM (200 mL) and the reaction mixture was stirred at rt. overnight. The solvent was removed to dryness to give the title compound (47.2 g, 100% yield), that was used in the next step without further purification.HPLC-MS (Method A): Ret, 2.34 mm; ESl-MS m/z, 262 (M+1).
References:
WO2017/16669,2017,A1 Location in patent:Page/Page column 201

100-39-0
438 suppliers
$10.00/10g

10429-82-0
159 suppliers
$7.00/1g