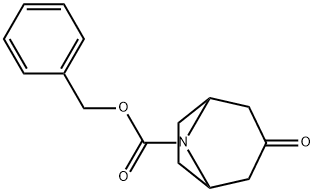
N-Cbz-Nortropinone synthesis
- Product Name:N-Cbz-Nortropinone
- CAS Number:130753-13-8
- Molecular formula:C15H17NO3
- Molecular Weight:259.3
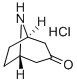
25602-68-0

501-53-1

130753-13-8
1. 8-Azabicyclo[3,2,1]octan-3-one hydrochloride (10.0 g; 71.84 mmol) was dissolved in dichloroethane (DCE, 60 mL), and 1-chloroethyl chloroformate (ACE-C1, 14.5 mL; 19.11 g; 133.7 mmol) was added slowly and dropwise. The reaction mixture was stirred overnight at room temperature, then diluted with ether (Et2O, 400 mL) and filtered. The filtrate was concentrated under reduced pressure to give crude chloroethyl carbamate. 2. The above crude product was dissolved in methanol (MeOH, 200 mL) and stirred at room temperature for 1 h. Subsequently, it was concentrated under reduced pressure at 55 °C to obtain crude desmethyltropinone hydrochloride (brown solid, 11.4 g, 98% yield). 3. The crude product was purified by recrystallization from formonitrile to give a white crystalline solid (5 g, 43% yield).1H NMR (400 MHz, DMSO-d6) δ 1.79 (dd, J = 15.0,6.9 Hz, 2H), 2.09 (m, 2H), 2.40 (d, J = 16.7 Hz, 2H), 3.02 (dd, J = 17.1,4.3 Hz, 2H), 4.23 (s, 2H), 10.00 (br s, 2H). 4. desmethyltropinone (5.10 g; 31.55 mmol) was dissolved in dichloromethane (CH2Cl2, 50 mL) and benzyl chloroformate (4.29 mL; 5.11 g; 29.98 mmol) and diisopropylethylamine (DIPEA, 16.48 mL; 12.23 g; 94.66 mmol) were added slowly and dropwise (note exothermic reaction). The reaction mixture was stirred at room temperature for 30 min and then diluted with dichloromethane (100 mL). 5. The organic phase was washed with 1N hydrochloric acid (2 x 100 mL), dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to give the crude product (7.2 g, 88% yield).1H NMR (400 MHz, CDCl3) δ 1.71 (dd, J = 15.0,7.2Hz, 2H), 2.12 (m, 2H), 2.38 (d, J = 15.9 Hz, 2H), 2.67 (m, 2H), 4.62 (s, 2H), 5.22 (s, 2H), 7.38 (m, 5H).

25602-68-0
212 suppliers
$11.00/5g

501-53-1
416 suppliers
$10.00/1g

130753-13-8
132 suppliers
$13.00/1g
Yield:130753-13-8 88%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 0.5 h;
Steps:
F
Tropinone (10.0 g; 71.84 mmol) was dissolved in DCE (60 mL) and treated drop-wisewith 1-chloroethyl chloroformate ACE-C1 (14.5 mL; 19.11 g; 133.7 mmol). The reaction wasallowed to stir at room temperature overnight and was then diluted with Et20 (400 mL) andfiltered. The filtrate was concentrated under reduced pressure to provide the crude chloroethylcarbamate. This compound was taken in MeOH (200 mL) and stirred at room temperature for 1h, then concentrated under reduced pressure (at 55°C) to provide the crude des-methyltropinoneas the HC1 salt (tan solid, 11.4 g, 98% yield). The crude material was recrystallized fromacetonitrile to furnish the pure product as a white crystalline solid (5 g, 43% yield). *H NMR(400 MHz, DMSO-d6) 8 1.79 (dd, J= 15.0, 6.9 Hz, 2H), 2.09 (m, 2H), 2.40 (d, J= 16.7 Hz,2H), 3.02 (dd, J= 17.1, 4.3 Hz, 2H), 4.23 (s, 2H), 10.00 (br s, 2H)Des-methyl tropinone (5.10 g; 31.55 mmol) was dissolved in CH2CI2 (50 mL) and treated withbenzyl chloroformate (4.29 mL; 5.11 g; 29.98 mmol) DIPEA (16.48 mL; 12.23 g; 94.66 mmol)was added drop-wise (exothermic reaction). The resulting clear solution was allowed to stir atroom temperature for 30 min and was subsequently diluted with 100 mL CH2CI2. The organicphase was washed with 1 N HC1 (2 x 100 mL), dried on Na2SC>4 and concentrated to provide thecrude product (7.2 g, 88% yield). *H NMR (400 MHz, CDC13) 8 1.71 (dd, J= 15.0, 7.2 Hz, 2H),2.12 (m, 2H), 2.38 (d, J= 15.9 Hz, 2H), 2.67 (m, 2H), 4.62 (s, 2H), 5.22 (s, 2H), 7.38 (m, 5H).
References:
WO2006/23852,2006,A2 Location in patent:Page/Page column 84

5632-84-8
53 suppliers
$198.00/1g

501-53-1
416 suppliers
$10.00/1g

130753-13-8
132 suppliers
$13.00/1g

532-24-1
399 suppliers
$9.00/10g

501-53-1
416 suppliers
$10.00/1g

130753-13-8
132 suppliers
$13.00/1g
![3-EXO-BENZYL 3-HYDROXY-8-AZABICYCLO[3.2.1]OCTANE-8-CARBOXYLATE](/CAS/20200401/GIF/96951-89-2.gif)
96951-89-2
3 suppliers
inquiry

130753-13-8
132 suppliers
$13.00/1g
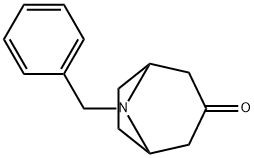
28957-72-4
216 suppliers
$28.00/1g

130753-13-8
132 suppliers
$13.00/1g