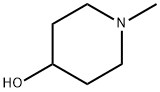
N-Methyl-4-piperidinol synthesis
- Product Name:N-Methyl-4-piperidinol
- CAS Number:106-52-5
- Molecular formula:C6H13NO
- Molecular Weight:115.17

1445-73-4

106-52-5
The general procedure for the synthesis of 1-methyl-4-piperidinol from N-methyl-4-piperidone was as follows: in standard experiments, the catalyst complex 1 (6.1 mg, 10 mol%) and the hydrogenation reagent H[BAr'4]-(Et2O)2 (10.1 mg, 10 mol%) were dissolved in a 100 mL thick-walled glass reactor fitted with a TEFLON stopper and stirring bar of THF (2.0 mL). Subsequently, the substrate to be hydrogenated (0.5 mmol) was added. The reaction system was degassed by a freeze-pump-thaw cycle and then charged with hydrogen (1 or 4 atm). The reaction mixture was stirred at a set temperature (25-60 °C) until the reaction was complete. At the end of the reaction, the solvent was removed by evaporation and the residue was initially purified by passing through a silica gel column. After removal of solvent under reduced pressure, the crude product was dissolved in CDCl3 and analyzed by 1H NMR. The final product was further purified by column chromatography or preparative thin layer chromatography (TLC) using hexane/ethyl acetate (3:1, v/v) as eluent. The purified products were characterized by 1H NMR and GC-MS, and the spectral data were consistent with those reported in the literature or real samples.
Yield:106-52-5 89%
Reaction Conditions:
with hydrogenchloride;palladium 10% on activated carbon;hydrogen in methanol;water; pH=2 - 3;
Steps:
1.1 Step 1) 1-methyl-4-piperidinol
A mixture of 4-piperidinol (1.16g, 10.07mmol) and 47% aqueous formaldehyde solution (10 mL) were dissolved in methanol (10 mL), and acidified with concentrated hydrochloricTo pH 2-3, to the system was added 10% Pd / C (300mg), two days the reaction system was kept under a hydrogen atmosphere. Completion of the reaction, filtered and the filtrate reducedPressure concentrated and the crude product purified by column chromatography (MeOH) to give a white solid (1.59g, 89%)
References:
CN105367582,2016,A Location in patent:Paragraph 0253; 0254; 0255

1445-73-4
445 suppliers
$15.00/25g

106-52-5
343 suppliers
$6.00/25g

695-19-2
13 suppliers
$305.00/500mg

106-52-5
343 suppliers
$6.00/25g

1445-73-4
445 suppliers
$15.00/25g
62-53-3
689 suppliers
$10.00/1g

106-52-5
343 suppliers
$6.00/25g

22261-94-5
32 suppliers
$34.79/1g

34737-83-2
35 suppliers
inquiry

106-52-5
343 suppliers
$6.00/25g

