
N-Succinimidyl maleimidoacetate synthesis
- Product Name:N-Succinimidyl maleimidoacetate
- CAS Number:55750-61-3
- Molecular formula:C10H8N2O6
- Molecular Weight:252.18
6066-82-6

17686-36-1

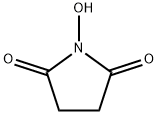
55750-61-3
GENERAL STEPS: To a 500 mL three-necked flask was added N-maleimidoacetic acid (94 g, 0.6 mol) and 300 mL of methylene chloride, followed by oxalyl chloride (90 g, 0.72 mol). The reaction mixture was heated to reflux and a large amount of gas was observed to be released. After 2 hours of reaction, the solution became clarified. Subsequently, dichloromethane and excess oxalyl chloride were removed by concentration. 200 mL of dichloromethane was added to wash and remove the residual acid to give the crude product N-maleimidoacetyl chloride. The crude product was dissolved in 200 mL of dichloromethane. In another vessel, N-hydroxysuccinimide (69 g, 0.6 mol) and triethylamine (80.8 g, 0.8 mol) were dissolved in 300 mL of dichloromethane and the reaction temperature was controlled to be no more than 20° C. under the cooling of an ice water bath. A dichloromethane solution of the above N-maleimidoacetyl chloride was slowly added dropwise to this mixture and the dropwise process lasted for 2 hours. Upon completion of the reaction, the reaction solution was washed with 200 mL of water to remove the triethylamine hydrochloride, followed by 100 mL of 1N hydrochloric acid to remove excess triethylamine, and finally with 200 mL of saturated brine. The organic phase was dried and concentrated to dryness to give 137 g of white solid. Purification by recrystallization in a mixed solvent of ethyl acetate and petroleum ether resulted in 116 g of white solid with 98.7% HPLC purity and 76.7% yield.

6066-82-6
791 suppliers
$5.00/10g

17686-36-1
1 suppliers
inquiry

55750-61-3
158 suppliers
$35.00/5mg
Yield: 116 g
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 2 h;
Steps:
7 Example 7
Synthesis of succinimidyl N-maleimidyl acetate, a compound of formula (V) wherein X is 1.
To a 500 ml three-necked flask, N-maleimidyl acetic acid (94 g, 0.6 mol)And dichloromethane 300ml,Oxalyl chloride (90 g, 0.72 mol) was then added,The reaction was warmed to reflux, a large amount of gas was released.After 2h the reaction became clear,Concentrate to remove methylene chloride and excess oxalyl chloride,Addition of 200 ml of methylene chloride to remove the residual acid in one pass afforded crude N-maleimidoacetyl chloride.The crude product was dissolved in 200 ml of dichloromethane,Was added dropwise to N-hydroxysuccinimide (69 g, 0.6 mol)Triethylamine (80.8 g, 0.8 mol)Dissolved in 300 ml of dichloromethane,Ice water cooling control temperature does not exceed 20 , drip finished room temperature 2h.The reaction solution was washed with 200 ml of water to remove triethylamine hydrochloride,1N hydrochloric acid 100ml washing to remove excess triethylamine,200ml saturated salt water, washed and dried,Concentrated to dry off like white solid 137g,After recrystallization from a mixed solvent of ethyl acetate and petroleum ether, 116 g of a white solid was obtained,HPLC purity 98.7%, 76.7% yield.
References:
Suzhou Haofan Biological Technology Co., Ltd.;Lv Minjie;Zhang Haiyan;Wang Guichun;Sun Fangchao CN105037237, 2017, B Location in patent:Paragraph 0071-0074

6066-82-6
791 suppliers
$5.00/10g

25021-08-3
145 suppliers
$27.00/100mg

55750-61-3
158 suppliers
$35.00/5mg

6066-82-6
791 suppliers
$5.00/10g

54930-24-4
35 suppliers
inquiry

55750-61-3
158 suppliers
$35.00/5mg

25021-08-3
145 suppliers
$27.00/100mg

55750-61-3
158 suppliers
$35.00/5mg
![2-Butenoic acid, 4-[(carboxymethyl)amino]-4-oxo-](/CAS/20210305/GIF/329709-87-7.gif)
329709-87-7
0 suppliers
inquiry

55750-61-3
158 suppliers
$35.00/5mg