
Pyridine, 5-(bromomethyl)-2-chloro- (9CI) synthesis
- Product Name:Pyridine, 5-(bromomethyl)-2-chloro- (9CI)
- CAS Number:182924-36-3
- Molecular formula:C6H5BrClN
- Molecular Weight:206.47

22536-61-4

182924-36-3
The general procedure for the synthesis of 5-bromomethyl-2-chloropyridine using 2-chloro-5-methylpyrimidine as starting material was as follows: synthetic embodiment 25, N-[1-((2-chloropyrimidin-5-yl)methyl)pyridin-2(1H)-ylidene]-2,2,2-trifluoroacetamide (compound 243). 2-Chloro-5-methylpyrimidine (1.04 g, 8.13 mmol) was dissolved in 30 mL of carbon tetrachloride and N-bromosuccinimide (1.73 g, 9.75 mmol) and benzoyl peroxide (20 mg) were added. The mixture was heated to reflux for 6 hours. Upon completion of the reaction, the reaction mixture was cooled to room temperature, concentrated under reduced pressure and purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 3:1) to afford 5-bromomethyl-2-chloropyridine 641 mg (38% yield).1H-NMR (CDCl3, δ, ppm): 4.42 (2H, s), 8.66 (2H, s).

22536-61-4
231 suppliers
$6.00/1g

182924-36-3
101 suppliers
$42.00/250mg
Yield: 38%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane for 6 h;Reflux;
Steps:
25
Synthesis Example 25
N-[1-((2-chloropyrimidin-5-yl)methyl)pyridin-2(1H)-ylidene]-2,2,2-trifluoroacetamide (Compound 243)
2-chloro-5-methylpyrimidine (1.04 g, 8.13 mmol) was dissolved in 30 mL of carbon tetrachloride, 1.73 g (9.75 mmol) of N-bromosuccinimide and 20 mg of benzoyl peroxide were added, and the mixture was refluxed under heating for 6 hours.
Following reaction completion, the reaction mixture was returned to room temperature, concentrated under reduced pressure, and purified by silica gel column chromatography (hexane/ethyl acetate=3:1), affording 641 mg of 5-bromomethyl-2-chloropyridine (yield, 38%).
1H-NMR (CDCl3, δ, ppm): 4.42 (2H, s), 8.66 (2H, s)
References:
MEIJI SEIKA PHARMA CO., LTD.;KAGABU, Shinzo;MITOMI, Masaaki;KITSUDA, Shigeki;HORIKOSHI, Ryo;NOMURA, Masahiro;ONOZAKI, Yasumichi US2013/150414, 2013, A1 Location in patent:Paragraph 0437

21543-49-7
258 suppliers
$8.00/1g

182924-36-3
101 suppliers
$42.00/250mg

18368-64-4
452 suppliers
$10.00/1g

182924-36-3
101 suppliers
$42.00/250mg

49608-01-7
269 suppliers
$7.00/5g

182924-36-3
101 suppliers
$42.00/250mg
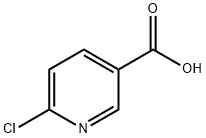
5326-23-8
562 suppliers
$5.00/1g

182924-36-3
101 suppliers
$42.00/250mg