
(R)-3-(Boc-Amino)piperidine synthesis
- Product Name:(R)-3-(Boc-Amino)piperidine
- CAS Number:309956-78-3
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28
485820-12-0

309956-78-3
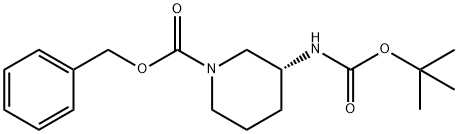
General procedure for the synthesis of (R)-3-Boc-aminopiperidine from benzyl (R)-3-((tert-butoxycarbonyl)amino)piperidine-1-carboxylate: 46.8 g of benzyl (R)-3-((tert-butoxycarbonyl)amino)piperidine-1-carboxylate (Compound IV), 2.3 g of 10% wet palladium-carbon (50% water) and 320 mL of methanol were added to an autoclave. The reaction system was replaced with nitrogen 3 to 4 times and the reaction temperature was raised to 35 to 40 °C under hydrogen pressure of 0.3 to 0.4 MPa and maintained for 2 hours. The progress of the reaction was monitored by HPLC until complete conversion of the feedstock. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated to be free of fractions. To the concentrate, 23 mL of dichloromethane and 46 mL of petroleum ether were added and the temperature was raised to 35 to 40 °C. At this temperature, 325 mL of petroleum ether was slowly added and held for 1 hour. The reaction mixture was then slowly cooled to -5~0°C and the solid product was collected by filtration. The solid was dried at 40~45°C to give 26.7 g of white solid (R)-3-Boc-aminopiperidine in 95.4% yield.
454713-13-4
99 suppliers
$27.00/100mg

309956-78-3
555 suppliers
$11.00/5g
Yield:309956-78-3 97%
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen in ethanol at 20 - 25; under 150015 Torr; for 20 h;
Steps:
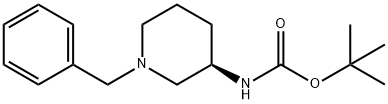
6 Example 6: Synthesis of R-3-(tert-butoxycarbonylamino)piperidine (7)
R-3-(tert-butoxycarbonylamino)-1-benzylpiperidine (20g) was dissolved in 96g of absolute ethanol, added with 5 mass% Pd/C (2g), passed into 20MPa hydrogen, and reacted at 20-25 °C. After 20 h, filtration and ethanol washing, the filtrate was combined and evaporated to dryness to give R-3-(tert-butoxycarbonylamino)piperidine (13.4 g), yield 97%.
References:
CN110078657,2019,A Location in patent:Paragraph 0135-0136

485820-12-0
47 suppliers
$90.00/50mg

309956-78-3
555 suppliers
$11.00/5g
![Carbamic acid, [(3R)-2-oxo-3-piperidinyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/221874-51-7.gif)
221874-51-7
64 suppliers
$33.00/100mg

309956-78-3
555 suppliers
$11.00/5g

24424-99-5
857 suppliers
$13.50/25G

309956-78-3
555 suppliers
$11.00/5g

16682-12-5
316 suppliers
$9.00/1g

309956-78-3
555 suppliers
$11.00/5g