
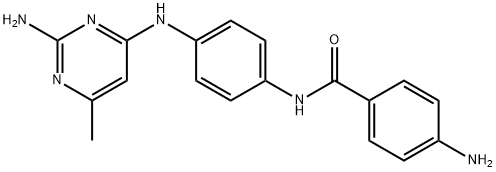
SGI-1027 synthesis
- Product Name:SGI-1027
- CAS Number:1020149-73-8
- Molecular formula:C27H23N7O
- Molecular Weight:461.52

20719-43-1

133041-90-4

1020149-73-8
GENERAL STEPS: 4-(quinolin-4-ylamino)benzoic acid (45 mg, 0.17 mmol) and N4-(4-aminophenyl)-6-methylpyrimidine-2,4-diamine (37 mg, 0.17 mmol) were dissolved in DMF (1.7 mL). Subsequently, N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (0.06 g, 0.34 mmol) and 1-hydroxybenzotriazole hydrate (0.03 g, 0.17 mmol) were added sequentially. The reaction mixture was stirred at 65oC for 24 hours. After completion of the reaction, the solvent was removed by vacuum concentration and the residue was purified by column chromatography (silica gel, eluent ratio 85:13:2 dichloromethane/methanol/triethylamine) to afford N-[4-[(2-amino-6-methyl-4-pyrimidinyl)amino]phenyl]-4-(4-quinolinylamino)benzamide (0.05 g, 59% yield) as an amorphous yellow solid.
611-35-8
214 suppliers
$5.00/250mg
1020150-22-4
8 suppliers
inquiry

1020149-73-8
99 suppliers
$29.00/1mg
Yield: 0.045 g
Reaction Conditions:
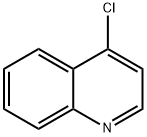
with palladium diacetate;potassium carbonate;XPhos in water;N,N-dimethyl-formamide;tert-butyl alcohol at 110; for 24 h;Sealed tube;Inert atmosphere;Buchwald-Hartwig Coupling;
Steps:
N-[4-(2-Amino-6-methylpyrimidin-4-ylamino)phenyl]-4-(quinolin-4-ylamino)benzamide, SGI-1027 (4).
A sealed tube was charged with Pd(OAc)2 (1 mg, 1.32 mmol) and XPhos (2 mg, 3.97 mmol) and degassed t-BuOH/water (1 mL, 2:1 v/v) was added. The resulting solution was heated for 1 min at 80 oC and then cannulated into another sealed tube charged with 4-amino-N-[4-(2-amino-6-methylpyrimidine-4-ylamino)phenyl]benzamide 12 (0.07g, 0.20 mmol), 4-chloroquinoline 13 (22.0 mg, 0.13 mmol) and potassium carbonate (0.03 g, 0.18 mmol) in DMF (1 mL). The resulting mixture was stirred for 24 h at 110 oC. When the reaction was complete the solvent was concentrated in vacuo and the residue was purified by column chromatography (silica gel, 85:13:2 CH2Cl2/MeOH/Et3N) to afford 0.045 g (73% by 1H-NMR) of the titled compound as an amorphous yellow solid. 1H-NMR (400.13 MHz, DMSO-d6): d 10.22 (s, 1H, CONH), 9.98 (s, 1H, NH), 8.59 (d, J = 5.6 Hz, 1H), 8.48 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 8.7 Hz, 2H), 8.00-7.94 (m, 2H), 7.86 - 7.75 (m, 3H), 7.74 - 7.61 (m, 4H), 7.57 - 7.50 (m, 3H), 7.44 - 7.37 (m, 1H), 7.15 (d, J = 5.6 Hz, 1H), 6.04 (s, 1H, H5’’), 2.22 (s, 3H, CH3) ppm. 13C-NMR (100.62 MHz, DMSO-d6): d 164.7 (s, CONH), 161.4 (s), 157.1 (s), 154.9 (s), 149.5 (s), 147.8 (d), 145.0 (s), 142.9 (s), 135.4 (s), 134.2 (s), 131.3 (d), 130.2 (s), 129.3 (d, 2x), 125.9 (d, 2x), 123.0 (d, 2x), 121.7 (d), 121.6 (d, 2x), 120.8 (d, 2x), 119.4 (s), 102.5 (d), 96.4 (d), 19.4 (q, CH3) ppm. HRMS (ESI+): Calcd for C27H24N7O ([M + H]+), 462.20368; found, 462.20330. IR (neat): n 3400-3000 (br, N-H), 2976 (w, C-H), 2891 (w, C-H), 1584 (m), 1510 (m), 1444 (m), 1380 (m), 1318 (m), 1228 (w), 1180 (w), 1095 (w) cm-1. UV (DMSO): lmax 258, 355 nm.
References:
García-Domínguez, Patricia;Dell'Aversana, Carmela;Alvarez, Rosana;Altucci, Lucia;De Lera, ángel R. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 6,p. 1631 - 1635] Location in patent:supporting information

5600-21-5
318 suppliers
$18.00/25g

1020149-73-8
99 suppliers
$29.00/1mg

100-01-6
65 suppliers
$11.19/25G

1020149-73-8
99 suppliers
$29.00/1mg

20719-42-0
0 suppliers
inquiry

1020149-73-8
99 suppliers
$29.00/1mg