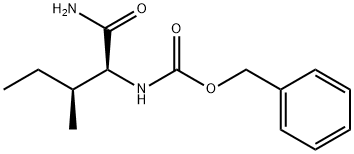
Z-ILE-NH2 synthesis
- Product Name:Z-ILE-NH2
- CAS Number:86161-49-1
- Molecular formula:C14H20N2O3
- Molecular Weight:264.32
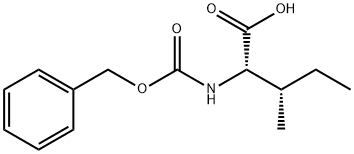
3160-59-6

3282-30-2

86161-49-1
11-1) Preparation of benzyl (2S,3S)-(1-amino-3-methyl-1-oxopentan-2-yl)carbamate 2.63 g (10 mmol) of (2S,3S)-2-(((benzyloxy)carbonyl)amino)-3-methylpentanoic acid was added to a 100 mL solution of dichloromethane pre-cooled to -20°C. 1.40 mL of triethylamine and 1.23 mL of tertiary chloroyl chloride were added sequentially. after 30 min, an ammonia gas bulge was passed into the reaction mixture for 30 min. The resulting precipitate was filtered and the solid was washed with 50 mL of methylene chloride. The combined organic phases were washed sequentially with 2N HCl solution (50 mL x 2) and 50 mL saturated brine. The organic layer was dried with anhydrous MgSO4 and concentrated in vacuum to give 2.49 g (2S,3S)-(1-amino-3-methyl-1-oxopentan-2-yl)carbamic acid benzyl ester (yield: 95%). 1H NMR (CDCl3) δ: 0.9 (m, 6H), 1.2 (m, 1H), 1.5 (m, 1H), 1.8 (m, 1H), 4.0 (m, 1H), 5.1 (s, 2H), 6.5 (d, 1H).

3160-59-6
256 suppliers
$10.00/1g

3282-30-2
380 suppliers
$22.00/100ml

86161-49-1
23 suppliers
$13.00/1g
Yield:86161-49-1 95%
Reaction Conditions:
with ammonia;triethylamine in dichloromethane;
Steps:
P.11.1 11-1)
11-1) Preparation of L-(N-benzyloxycarbonyl)-isoleucineamide To a pre-cooled solution(-20° C.) of 2.63 g(10 mmol) of L-(N-benzyloxycarbonyl)-isoleucine in 100 ml of dichloromethane were added 1.40 ml of triethylamine and 1.23 ml of pivaloyl chloride successively. After 30 minutes, ammonia gas was bubbled through the reaction mixture for 30 minutes. The resulting precipitate was filtered and the solid was washed with 50 ml of dichloromethane. The combined solution was washed successively with 2N HCl solution(50 ml*2) and 50 ml of brine. The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo to give 2.49 g of the title compound (yield: 95%). 1 H NMR(CDCl3) δ0.9(m, 6H), 1.2(m, 1H), 1.5(m, 1H), 1.8(m, 1H), 4.0(m, 1H), 5.1(s, 2H), 6.5(d, 1H)
References:
US5587388,1996,A
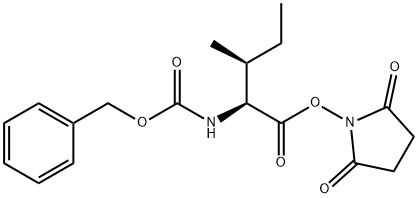
3391-99-9
70 suppliers
$23.30/5G

86161-49-1
23 suppliers
$13.00/1g

3160-59-6
256 suppliers
$10.00/1g

86161-49-1
23 suppliers
$13.00/1g