
Z-TRANS-4-HYDROXY-L-PROLINOL synthesis
- Product Name:Z-TRANS-4-HYDROXY-L-PROLINOL
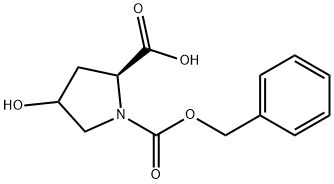
- CAS Number:95687-41-5
- Molecular formula:C13H17NO4
- Molecular Weight:251.28

13504-85-3

95687-41-5
N-Benzyloxycarbonyl-(4R)-hydroxy-L-proline (150 g, 0.565 mol) was used as a raw material, which was dissolved in tetrahydrofuran (1.5 L), and the borane-dimethyl sulfide complex (59.0 ml, 0.622 mol) was added dropwise slowly under stirring at 0 °C. After the dropwise addition, the reaction mixture was heated to reflux and stirred continuously for 3 hours. Subsequently, the reaction mixture was cooled to 0 °C and borane-dimethyl sulfide complex (16.1 ml, 0.170 mol) was added again and stirring was continued for 10 hours. After completion of the reaction, the reaction mixture was cooled and water (500 ml) was added slowly at 0 °C to quench the excess borane-dimethyl sulfide complex. The reaction mixture was extracted with a solvent mixture of ethyl acetate and chloroform-methanol (10:1, v/v), the organic phases were combined, washed with saturated sodium chloride solution and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to afford Cbz-(2S,4R)-4-hydroxyprolinol (122.06 g, 86% yield), the product was a pale yellow oil, which could be used for subsequent reactions without further purification.1H-NMR (CDCl3) δ: 1.52-1.81 (m, 3H), 2.06 (m, 1H), 3.40-3.85 and 4.04- 4.61 (m series, total 6H), 5.15 (s, 2H, ArCH2), 7.20-7.44 (m, 5H, Ar).

64187-48-0
154 suppliers
$14.00/5G

95687-41-5
136 suppliers
$19.00/250mg
Yield:95687-41-5 99%
Reaction Conditions:
with lithium tetrahydridoborate in tetrahydrofuran at 0 - 20; for 4.5 h;
Steps:
1.6
(2S, 4R)-N-LBeLzyloxvcarbonvo-2-hydroxymethyl-4-hydroxvpvrrolidine (6); To a stirred, cold (0 °C) solution of intermediate (5) (58.4 g, 0.208 mol) in THF (400 mL) was added LiBH4 (3.5 g, 0.16 mol) portion-wise. The reaction mixture was stirred for 30 min at (0-5 °C) then for 4 h at room temperature. At this time, TLC (silica gel plates developed in EtOAc) showed just a trace of starting material. The thick reaction mixture was diluted in succession with H20 (250 mL) and 2 M HC1 (277 mL), and then concentrated in vacuo to remove THF. The resulting aqueous concentrate was extracted with EtOAc (4 x 175 mL). Combined EtOAc extracts were washed with brine (2 x 200 mL), dried over MgS04, and then concentrated in vacuo to give intermediate (5) as an oil (51.9 g, 99%). Additional reactions were carried out to give a total of 227 g of product suitable for further transformation. In later preparations the more convenient commercial solution of LiBH4 in THF was used
References:
WO2005/40170,2005,A2 Location in patent:Page/Page column 33-34

13504-85-3
288 suppliers
$5.00/1g

95687-41-5
136 suppliers
$19.00/250mg
197079-48-4
0 suppliers
inquiry

95687-41-5
136 suppliers
$19.00/250mg

501-53-1
416 suppliers
$10.00/1g

95687-41-5
136 suppliers
$19.00/250mg

51-35-4
888 suppliers
$6.00/5g

95687-41-5
136 suppliers
$19.00/250mg