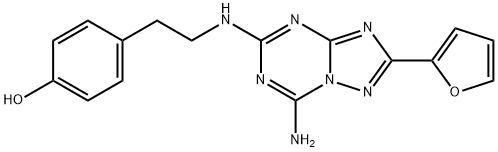
ZM 241385 synthesis
- Product Name:ZM 241385
- CAS Number:139180-30-6
- Molecular formula:C16H15N7O2
- Molecular Weight:337.34
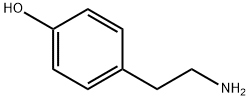
51-67-2
![2-(furan-2-yl)-5-(methylsulfonyl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amine](/CAS/20180529/GIF/139181-28-5.gif)
139181-28-5

139180-30-6
A reaction was carried out in acetonitrile (25 mL) with p-hydroxyphenethylamine (tyramine, 490 mg, 3.75 mmol) and 2-(furan-2-yl)-5-(methylsulfonyl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amine (250 mg, 892 μmol). The reaction mixture was stirred at room temperature for 22 hours. Upon completion of the reaction, the solvent was removed by evaporation and the residue was adsorbed through diatomaceous earth and purified using column chromatography (eluent: dichloromethane/methanol, 25:75, v/v). The target product grade was collected, evaporated to dryness and subsequently recrystallized from ethyl acetate to afford 4-(2-((7-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl)amino)ethyl)phenol (100 mg, 33% yield) as a white solid, melting point: 229-231 °C (literature value: 225- 227°C). The product was analyzed and confirmed by 1H NMR, 13C NMR, high resolution mass spectrometry (HRMS) and high performance liquid chromatography (HPLC).1H NMR (400 MHz, d6-DMSO, 353 K) δ 8.91 (s, 1H), 7.92 (br s, 2H), 7.79 (m, 1H), 7.14 (br t, 1H), 7.04 (m, 3H) , 6.71 (m, 2H), 6.64 (dd, J = 3.3, 1.8 Hz, 1H), 3.49 (m, 2H), 2.78 (m, 2H).13C NMR (101 MHz, d6-DMSO) δ 161.1 (C), 159.0 (C), 155.9 (C), 155.4 (C), 150.1 (C), 146.1 (C), 146.1 (C), 146.1 (C), 146.1 (C), 146.1 (C), 146.1 (C). 146.1 (C), 144.1 (CH), 129.1 (CH), 115.0 (CH), 111.4 (CH), 111.2 (CH), 42.4 (CH2), 33.9 (CH2). HRMS (C16H15N7O2): Calculated value 338.1360 [M + H]+, measured value 338.1353. HPLC : tR 7.34 min, purity 98% (214 nm), 97% (254 nm).

51-67-2
490 suppliers
$13.00/5g
![2-(furan-2-yl)-5-(methylsulfonyl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amine](/CAS/20180529/GIF/139181-28-5.gif)
139181-28-5
25 suppliers
inquiry

139180-30-6
115 suppliers
$25.00/1mg
Yield:139180-30-6 33%
Reaction Conditions:
in acetonitrile at 20;
Steps:
4-(2-((7-Amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl)amino)ethyl)phenol (5) (ZM 241385)
Tyramine (490 mg, 3.75 mmol) was added to a suspension of 2-(furan-2-yl)-5-(methylsulfonyl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amine (18) (250 mg, 892 μmol) in acetonitrile (25 mL). The reaction mixture was stirred overnight at room temperature. After 22 h the solvent was evaporated, the residue was adsorbed on Celite and purified by column chromatography (dichloromethane: methanol 25:75). The collected fractions with product were evaporated to dryness and recrystallised from ethyl acetate. The title compound 5 (100 mg, 33%) was obtained as a white solid, mp: 229-231 °C (lit.6 225-227 °C). 1H NMR (400 MHz, d6-DMSO, 353 K) δ 8.91 (s, 1H), 7.92 (br s, 2H), 7.79 (m, 1H), 7.14 (br t, 1H), 7.04 (m, 3H), 6.71 (m, 2H), 6.64 (dd, J = 3.3, 1.8 Hz, 1H), 3.49 (m, 2H), 2.78 (m, 2H). 13C NMR (101 MHz, d6-DMSO) δ 161.1 (C), 159.0 (C), 155.9 (C), 155.4 (C), 150.1 (C), 146.1 (C), 144.1 (CH), 129.1 (CH), 115.0 (CH), 111.4 (CH), 111.2 (CH), 42.4 (CH2), 33.9 (CH2). HRMS (C16H15N7O2): calc’d 338.1360 [M+H]+, found 338.1353. HPLC: tR 7.34 min, 98% (214 nm), 97% (254 nm).
References:
J?rg, Manuela;Shonberg, Jeremy;Mak, Frankie S.;Miller, Neil D.;Yuriev, Elizabeth;Scammells, Peter J.;Capuano, Ben [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 11,p. 3427 - 3433] Location in patent:supporting information
![[(2-furanylcarbonyl)amino]-](/CAS/20180527/GIF/6053-32-3.gif)
6053-32-3
1 suppliers
inquiry

139180-30-6
115 suppliers
$25.00/1mg

3663-61-4
40 suppliers
$45.00/10mg

139180-30-6
115 suppliers
$25.00/1mg
3326-71-4
223 suppliers
$15.00/5mg

139180-30-6
115 suppliers
$25.00/1mg

139181-27-4
12 suppliers
inquiry

139180-30-6
115 suppliers
$25.00/1mg