| Identification | More | [Name]
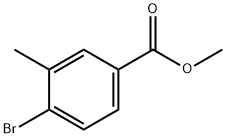
Methyl 4-bromo-3-methylbenzoate | [CAS]
148547-19-7 | [Synonyms]
4-BROMO-3-METHYLBENZOIC ACID METHYL ESTER
4-BROMO-M-TOLUIC ACID METHYL ESTER
METHYL 4-BROMO-3-METHYLBENZOATE
METHYL 4-BROMO-M-TOLUATE
Methyl 4-Bromo-3-Methoxynicotinate | [EINECS(EC#)]
629-103-4 | [Molecular Formula]
C9H9BrO2 | [MDL Number]
MFCD00673014 | [Molecular Weight]
229.07 | [MOL File]
148547-19-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
38-44 °C (lit.) | [Boiling point ]
287.4±20.0 °C(Predicted) | [density ]
1.433±0.06 g/cm3(Predicted) | [Fp ]
>230 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
soluble in Methanol | [form ]
Low Melting Solid or Liquid | [color ]
White to yellow | [BRN ]
6593534 | [InChI]
InChI=1S/C9H9BrO2/c1-6-5-7(9(11)12-2)3-4-8(6)10/h3-5H,1-2H3 | [InChIKey]
GTZTYNPAPQKIIR-UHFFFAOYSA-N | [SMILES]
C(OC)(=O)C1=CC=C(Br)C(C)=C1 | [CAS DataBase Reference]
148547-19-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HS Code ]
29163990 |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless low melting solid | [Uses]
Methyl 4-bromo-3-methylbenzoate may be used in the preparation of:
- 4-bromo-3-vinylbenzoic acid
- 4-bromo-3-(methylphenyl)methanol
- methyl (E)-4-bromo-3-[(phenylmethoxyimino)methyl]benzoate
It may be used as a starting material in the total synthesis of (-)-martinellic acid. | [General Description]
Methyl 4-bromo-3-methylbenzoate, an aromatic ester, undergoes Suzuki coupling with 2-chloroboronic acid to afford the corresponding biaryl. | [Synthesis]
To a concentrated solution of 4-bromo-3-methylbenzoic acid (3 g, 13.19 mmol) in methanol (15 ml) was added concentrated sulfuric acid (catalytic amount). The reaction mixture was heated to reflux for 14 hours and subsequently cooled to 0°C. The reaction mixture was neutralized with saturated sodium bicarbonate solution and the precipitated solid product was collected by filtration. The crude product was purified by silica gel column chromatography to afford methyl 4-bromo-3-methylbenzoate (3.1 g, 97% yield) as a white solid. | [References]
[1] Patent: US2011/71150, 2011, A1. Location in patent: Page/Page column 33
[2] Angewandte Chemie - International Edition, 2008, vol. 47, # 44, p. 8482 - 8486
[3] Patent: WO2006/110173, 2006, A2. Location in patent: Page/Page column 78
[4] Chemical Communications, 2018, vol. 54, # 65, p. 9051 - 9054
[5] Patent: US2013/131016, 2013, A1. Location in patent: Paragraph 0189 |
|
|