| Identification | More | [Name]
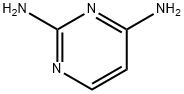
2,4-Diaminopyrimidine | [CAS]
156-81-0 | [Synonyms]
2,4-DIAMINOPYRIMIDINE
2,4-PYRIMIDINEDIAMINE
2,6-DIAMINOPYRIMIDINE
DAP
Pyrimidine,2,4-diamino-
Pyrimidine-2,4-diamine
3-Morpholinosydnoneimine HCl
pyrimidine-2,4-diyldiamine
Diaminopyrimidine
2,4-Pyrimidinediamine (9CI)
2,4-Diaminopyrimidine (sulphate)
2,4-PYRIMIDINEDIAMINE 98+%
2,4-DIAMINOPYRIMIDINE 97% | [EINECS(EC#)]
205-862-3 | [Molecular Formula]
C4H6N4 | [MDL Number]
MFCD00038023 | [Molecular Weight]
110.12 | [MOL File]
156-81-0.mol |
| Chemical Properties | Back Directory | [Appearance]
light orange crystalline powder | [Melting point ]
143-147 °C (lit.) | [Boiling point ]
401.7±37.0 °C(Predicted) | [density ]
1.368±0.06 g/cm3(Predicted) | [refractive index ]
1.46-1.466
| [storage temp. ]
Refrigerator (+4°C) | [form ]
powder to crystal | [pka]
7.08±0.10(Predicted) | [color ]
White to Almost white | [Water Solubility ]
840 g/L (25 ºC) | [InChI]
InChI=1S/C4H6N4/c5-3-1-2-7-4(6)8-3/h1-2H,(H4,5,6,7,8) | [InChIKey]
YAAWASYJIRZXSZ-UHFFFAOYSA-N | [SMILES]
C1(N)=NC=CC(N)=N1 | [CAS DataBase Reference]
156-81-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [HS Code ]
29335990 |
| Hazard Information | Back Directory | [Chemical Properties]
light orange crystalline powder | [Definition]
ChEBI: Pyrimidine-2,4-diamine is an aminopyrimidine in which a pyrimidine nucleus is substituted with amino groups at C-2 and C-4. | [Synthesis]
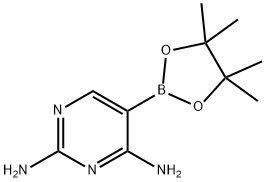
Synthesis of 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine-2,4-diamine: To a dry 1L flask was added 5-bromopyrimidine-2,4-diamine (30.0 g, 158.7 mmol), potassium acetate (45.8 g, 466.7 mmol), pinacol bis(boronic acid) ester (51.16 g, 202.2 mmol) and dioxane (500 mL). After bubbling argon through the solution for 15 minutes, 1,1'-bis(diphenylphosphino)ferrocene palladium(II) chloride (2.53 g, 3.11 mmol) was added. The reaction mixture was refluxed in an oil bath at 115 °C for 16 h under argon protection. After completion of the reaction, it was cooled to room temperature, filtered to remove solid inorganic material and washed with ethyl acetate (1 L). The organic filtrate was concentrated under vacuum and dichloromethane (1 L) was added to the resulting solid. After sonication, the solid was collected by filtration and this solid was the debromination product 2,4-diaminopyrimidine. The filtrate containing the target borate was concentrated in vacuo and ether (100 mL) was added to the residue. After sonication, the filtrate was filtered and washed with additional ether (50 mL), and the resulting solid was dried under high vacuum to afford the target product 2,4-diaminopyrimidine-5-boronic acid pinacol ester (10.13 g, 27% yield). The substance was analyzed by 1H NMR as a 4:1 mixture of 2,4-diaminopyrimidine-5-boronic acid pinacol ester and 2,4-diaminopyrimidine by-product. The mixture could be used in the subsequent Suzuki reaction without further purification.LCMS (m/z): 155 (MH+ of boronic acid, derived from hydrolysis of the in situ product on LC); 1H NMR (CDCl3 + CD3OD): δ8.16 (s, 1H), 1.34 (s, 12H). | [References]
[1] Patent: WO2008/98058, 2008, A1. Location in patent: Page/Page column 64-65
[2] Patent: WO2007/84786, 2007, A1. Location in patent: Page/Page column 98
[3] ACS Medicinal Chemistry Letters, 2011, vol. 2, # 10, p. 774 - 779
[4] Patent: WO2012/109423, 2012, A1. Location in patent: Page/Page column 23 |
|
|