| Identification | More | [Name]
3-(4-Chlorophenyl)glutaric acid | [CAS]
35271-74-0 | [Synonyms]
3-(4-CHLOROPHENYL)GLUTARIC ACID
3-(4-CHLOROPHENYL)-PENTANEDIOIC ACID
B-(4-CHLOROPHENYL) GLUTARIC ACID
BETA-(4-CHLOROPHENYL)GLUTARIC ACID
CBI-BB ZERO/006271
-(4-Chlorophenyl)glutaricacid
3-(4-chlorophenyl) glutarate
b-(p-Chlorophenyl)glutaric Acid
-(p-Chlorophenyl)glutaric Acid | [EINECS(EC#)]
252-477-1 | [Molecular Formula]
C11H11ClO4 | [MDL Number]
MFCD00190249 | [Molecular Weight]
242.66 | [MOL File]
35271-74-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
164-166°C | [Boiling point ]
394.4±27.0 °C(Predicted) | [density ]
1.396±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Soluble in dimethyl sulfoxide. Slightly soluble in methanol. | [form ]
Solid | [pka]
4.01±0.10(Predicted) | [color ]
White to Light Yellow | [BRN ]
1976828 | [InChI]
InChI=1S/C11H11ClO4/c12-9-3-1-7(2-4-9)8(5-10(13)14)6-11(15)16/h1-4,8H,5-6H2,(H,13,14)(H,15,16) | [InChIKey]
URXVLIVRJJNJII-UHFFFAOYSA-N | [SMILES]
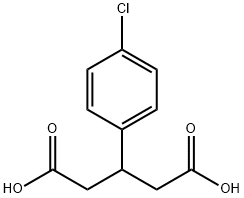
C(O)(=O)CC(C1=CC=C(Cl)C=C1)CC(O)=O | [CAS DataBase Reference]
35271-74-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [HazardClass ]
IRRITANT |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
3-(4-Chlorophenyl)glutaric Acid (cas# 35271-74-0) is a compound useful in organic synthesis. Baclofen Impurity 1 | [Synthesis]
The general procedure for the synthesis of 3-(4-chlorophenyl)glutaric acid using baclofen impurity 9 as starting material: the intermediate Π was dissolved in 420 mL of a 30 N aqueous solution of potassium hydroxide and the reaction was stirred for 2 hr at 85-90 °C. The reaction process was monitored by thin layer chromatography (TLC) and the unfolding agent was a mixture of ethyl acetate and petroleum ether (2:3, v/v). After completion of the reaction, the reaction mixture was cooled to room temperature and 400 mL of dichloromethane and 500 mL of deionized water were added sequentially. After stirring for 20 min, the organic and aqueous layers were separated and the organic layer was discarded. The aqueous layer was acidified to pH 1-2 with 6 M hydrochloric acid and the precipitated solid was subsequently filtered. The solid was washed with deionized water and dried under vacuum at 55-60°C for 5 h to give 157 g of white solid intermediate in 86.4% yield. The melting point of the intermediate was 166.5~167.3 °C. | [References]
[1] Patent: CN106187794, 2016, A. Location in patent: Paragraph 0023; 0028 |
|
|




![Butanoic acid, 2-[(4-chlorophenyl)methylene]-3-oxo-, ethyl ester](/CAS/20210305/GIF/19411-80-4.gif)
