| Identification | More | [Name]
alpha-D-Glucose pentaacetate | [CAS]
3891-59-6 | [Synonyms]
1,2,3,4,6-PENTA-O-ACETYL-D-GLUCOPYRANOSE
A,B-D-GLUCOSE PENTAACETATE
ALPHA, BETA-D-GLUCOSE PENTAACETATE
ALPHA, BETA-D-(+)-GLUCOSE PENTAACETATE (HIGH BETA)
D-GLUCOSE PENTAACETATE
2,3,4,5,6-Penta-O-acetylhexose
alpha-d-Glucose 2,3,4,5,6-pentaacetate
d-Allose, pentaacetate(ester)
Glucose acetate
Gulose pentaacetate
Pentaacetylglucose
Beta-D-GlucosePentaacetate98%
Beta-D-Glucose Pentaacetate 98%
ALPHA,BETA-D-GLUCOSEPENTAACETATE(HIGHALPHA)
EINECS 223-439-1
alpha-D-Glucose pentaacetate
D-Glucose 2,3,4,5,6-pentaacetate
D-Glucose pentaacetate ,98% [Sum of α, β enantiomers] | [EINECS(EC#)]
223-439-1 | [Molecular Formula]
C16H22O11 | [MDL Number]
MFCD00080787 | [Molecular Weight]
390.34 | [MOL File]
3891-59-6.mol |
| Chemical Properties | Back Directory | [Melting point ]
110°C | [Boiling point ]
435.58°C (rough estimate) | [density ]
1.3984 (rough estimate) | [FEMA ]
2524 | GLUCOSE PENTAACETATE | [refractive index ]
1.5376 (estimate) | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [Appearance]
White to off-white Solid | [Odor Type]
odorless | [Water Solubility ]
1.5g/L(18 ºC) | [Detection Methods]
NMR,MP,Rotation | [InChI]
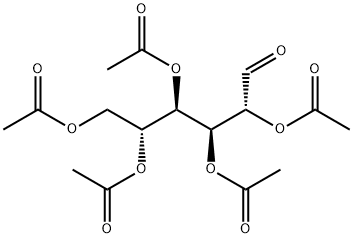
InChI=1S/C16H22O11/c1-8(18)23-7-14(25-10(3)20)16(27-12(5)22)15(26-11(4)21)13(6-17)24-9(2)19/h6,13-16H,7H2,1-5H3/t13-,14+,15+,16+/m0/s1 | [InChIKey]
UAOKXEHOENRFMP-ZJIFWQFVSA-N | [SMILES]
O=C[C@H](OC(=O)C)[C@@H](OC(=O)C)[C@H](OC(=O)C)[C@H](OC(=O)C)COC(=O)C | [LogP]
1.27 | [CAS DataBase Reference]
3891-59-6(CAS DataBase Reference) | [NIST Chemistry Reference]
D-Glucose, 2,3,4,5,6-pentaacetate(3891-59-6) | [EPA Substance Registry System]
3891-59-6(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Glucose pentaacetate is odorless, but has a bitter favor. | [Chemical Properties]
White solid | [Preparation]
By acetylation of glucose using any number of techniques, including ZnCl2 and pyridine, sodium acetate, and acetic anhydride and pyridine. | [Taste threshold values]
Taste characteristics at 100 ppm: bitter-like with a citrus tonic favor. | [Synthesis]
General procedure for the synthesis of 2,3,4,5,6-D-glucose pentaacetate from D-anhydrous glucose and acetic anhydride: D-glucose (0.36 g; 2 mmol) was dissolved in N,N'-dimethylimidazolium or N,N'-butylmethyl H-phosphonate methyl ester (1 g) at room temperature, followed by slow addition of acetic anhydride (1.02 g; 10 mmol). Continuous stirring is required during the reaction, noting that the reaction is exothermic and the mixture will gradually become homogeneous. The reaction can be continued at room temperature for 15 hours or accelerated at 50 °C to completion in 5 hours. At the end of the reaction, the target product 2,3,4,5,6-D-glucose pentaacetate is precipitated by the addition of water (0.72 g) to give a final yield of 92%. | [References]
[1] Patent: US2010/121075, 2010, A1. Location in patent: Page/Page column 8
[2] Heterocycles, 1987, vol. 26, # 6, p. 1549 - 1556
[3] Bulletin of the Chemical Society of Japan, 2010, vol. 83, # 7, p. 799 - 801
[4] Journal of Natural Products, 2011, vol. 74, # 8, p. 1812 - 1816 |
|
|