| Identification | More | [Name]
Ethyl (trimethylsilyl)acetate | [CAS]
4071-88-9 | [Synonyms]
ETHYL(2-TRIMETHYLSILYL)ACETATE
ETHYL (TRIMETHYLSILYL)ACETATE
ETSA
(TRIMETHYLSILYL)ACETIC ACID ETHYL ESTER
Acetic acid, (trimethylsilyl)-, ethyl ester
Ethyl(Trimethylsily)Acetate
ETSA~(Trimethylsilyl)acetic acid ethyl ester
ETHYL TRI METHYLSILYLACETATE 99%
Ethyl (trimethylsilyl)acetate, 98+%
2-(Trimethylsilyl)acetic acid ethyl ester
2-[Dimethyl(methyl)silyl]acetic acid ethyl ester | [EINECS(EC#)]
223-783-2 | [Molecular Formula]
C7H16O2Si | [MDL Number]
MFCD00009172 | [Molecular Weight]
160.29 | [MOL File]
4071-88-9.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless liquid | [Boiling point ]
156-159 °C (lit.) | [density ]
0.876 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.415(lit.)
| [Fp ]
95 °F
| [storage temp. ]
Flammables area | [solubility ]
sol ethereal and chlorinated solvents; reacts with
protic solvents. | [form ]
clear liquid | [color ]
Colorless to Almost colorless | [Specific Gravity]
0.876 | [Water Solubility ]
Decomposition | [Hydrolytic Sensitivity]
2: reacts with aqueous acid | [Sensitive ]
Moisture Sensitive | [BRN ]
1755902 | [CAS DataBase Reference]
4071-88-9(CAS DataBase Reference) | [NIST Chemistry Reference]
Ethyl (trimethylsilyl)acetate(4071-88-9) |
| Safety Data | Back Directory | [Risk Statements ]
R10:Flammable. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 3272 3/PG 3
| [WGK Germany ]
3
| [F ]
10-21 | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless liquid | [Physical properties]
bp 157–158 °C; d 0.876 g cm?3. | [Uses]
(silylating agent; source of an ethyl acetate anion equivalent.
Ethyl trimethylsilylacetate is reactive to nucleophiles and
readily undergoes desilylation reactions with acid or alkali,
ethanol, and bromine .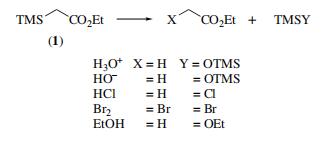
| [Uses]
Ethyl trimethylsilylacetate is used in the synthesis of ethyl-2,2-dibromo-2-(trimethylsilyl)acetate. It was also used to silylate the enolizable aldehydes and ketones. | [Preparation]
Available by a Reformatsky reaction from
ethyl bromoacetate, and by reaction of trimethylsilylmethyl�magnesium chloride with ethyl chloroformate. An alternative
approach requires the treatment of ethyl acetate with triphenylmethylsodium followed by chlorotrimethylsilane The use
of a nitrogen base with ethyl acetate in THF followed by
reaction with chlorotrimethylsilane results in a mixture of
C- and O-silylation. The use of HMPA as additive in the reaction medium increases the amount of O-silylation to 90%.
Similar methods can be used to prepare analogs. | [General Description]
Reaction of ethyl trimethylsilylacetate (ETSA) with dilute hydrochloric acid, dilute alkali, anhydrous HCl, anhydrous bromine and absolute ethanol has been reported. ETSA undergoes condensation with aromatic aldehydes in the presence of base catalyst to yield β-trimethylsiloxycarboxylates. Lithium ETSA reacts with ketones to yield α,β-unsaturated esters. | [Synthesis]
Zinc powder (9.92 g, 152 mmol) and copper(I) chloride (1.50 g, 15.2 mmol) were added to 15 mL of dry benzene under nitrogen protection, stirred and refluxed for 30 min. Subsequently, trimethylchlorosilane (12.8 mL, 101 mmol) and ethyl bromoacetate (12.3 mL, 111 mmol) dissolved in a mixture of 23 mL of anhydrous tetrahydrofuran and 21 mL of anhydrous benzene were slowly added through a dropping funnel, maintaining a mild reflux condition. The titration process took about 1 hour. After dropwise addition, reflux was continued for 1 hour, followed by cooling in an ice bath. Under stirring, 30 mL of 5% aqueous hydrochloric acid solution was added through a dropping funnel in 10 minutes. The liquid layer was separated and the reaction flask was washed with ether. The organic phase was combined and washed sequentially with saturated sodium bicarbonate solution twice, saturated sodium chloride solution twice, then saturated sodium bicarbonate solution twice, and finally saturated sodium chloride solution twice. The combined organic layers were dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give the crude product. Purification by reduced pressure distillation (boiling point 93-94 °C, 104 mmHg) gave ethyl 2-(trimethylsilyl)acetate as a pale yellow liquid in 74% yield (12.0 g, 74.9 mmol).1H NMR (CDCl3) δ 4.09 (q, J = 7.1 Hz, 2H), 1.89 (s, 2H), 1.24 (t, J = 7.1 Hz. 3H), 0.12 (s, 9H). | [Purification Methods]
Purify it by distilling ca 10g of reagent through a 15cm, Vigreux column (p 11) and then redistilling it through a 21cm glass helices-packed column [Hauze & Hauser J Am Chem Soc 75 994 1953]. Alternatively, dissolve it in Et2O, wash with H2O, dilute Na2CO3, dry over Na2CO3, evaporate Et2O, and distil it through a column of 15 theoretical plates. [Gold et al. J Am Chem Soc 70 2874 1948, Beilstein 4 IV 3974.] | [References]
[1] Chemistry Letters, 2017, vol. 46, # 2, p. 228 - 231 |
|
|