| Identification | Back Directory | [Name]
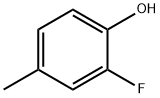
2-FLUORO-4-METHYLPHENOL | [CAS]
452-81-3 | [Synonyms]
2-Fluoro-p-cresol
2-FLUORO-4-METHYLPHENOL
Phenol, 2-fluoro-4-methyl-
3-Fluoro-4-hydroxytoluene, 2-Fluoro-p-cresol | [Molecular Formula]
C7H7FO | [MDL Number]
MFCD03094332 | [MOL File]
452-81-3.mol | [Molecular Weight]
126.13 |
| Chemical Properties | Back Directory | [Boiling point ]
173.0±20.0 °C(Predicted) | [density ]
1.164±0.06 g/cm3(Predicted) | [refractive index ]
1.513 | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [form ]
liquid | [pka]
9.01±0.18(Predicted) | [color ]
Clear, colourless | [InChI]
InChI=1S/C7H7FO/c1-5-2-3-7(9)6(8)4-5/h2-4,9H,1H3 | [InChIKey]
WJKISRFVKIOBCQ-UHFFFAOYSA-N | [SMILES]
C1(O)=CC=C(C)C=C1F |
| Hazard Information | Back Directory | [Synthesis]
GENERAL METHOD: To a solution of the substrate 4-methylphenol (1 mmol) in 10 mL of solvent (acetonitrile or methanol) was added Fluoride Reagent 1, 2 or 3 (1.1 mmol). The reaction mixture was stirred under reflux conditions for 2 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The crude product was dissolved in 50 mL of dichloromethane and filtered to remove insoluble material. The filtrate was washed with 30 mL of water and the organic layer was dried with anhydrous sodium sulfate followed by evaporation of the solvent. The crude product was analyzed by 1H NMR and 19F NMR spectroscopy. The relative distribution and total yield of the fluorinated products were determined by 19F NMR using octafluoronaphthalene (OFN) as an internal standard. The identification of the products was accomplished by comparing their NMR spectral data with those reported in the literature, independently prepared samples, or their conversion to known compounds. In the presence of excess reagents, 2-fluoro-4-alkyl-substituted phenols or alkoxybenzenes (5, see Scheme 1) can be converted to 2,2-difluoro-4-alkyl-3,5-cyclohexadienones (unknown compounds 8b or 8c) or hydrolyzed to 2-fluoro-4-methylphenols (4aa). 4-fluoro-4-alkyl-2,5-cyclohexadienones (6a, 6b, and 6c) were determined by preparative thin layer chromatography (silica gel , dichloromethane/petroleum ether, 4:1) from the crude mixture and identified on the basis of their spectral data. Also, 4-fluoroalkoxybenzene derivatives could be identified by hydrolytic conversion to 4-fluorophenol or by comparison with independently prepared compounds. | [References]
[1] Journal of Fluorine Chemistry, 1981, vol. 17, p. 159 - 172
[2] European Journal of Organic Chemistry, 2017, vol. 2017, # 17, p. 2469 - 2474
[3] Journal of Fluorine Chemistry, 1981, vol. 17, p. 159 - 172
[4] Journal of Fluorine Chemistry, 2013, vol. 156, p. 276 - 282
[5] Tetrahedron, 2006, vol. 62, # 18, p. 4474 - 4481 |
|
|