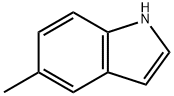
| Identification | More | [Name]
5-METHYLINDOLE-3-CARBOXALDEHYDE | [CAS]
52562-50-2 | [Synonyms]
5-METHYL-1H-INDOLE-3-CARBALDEHYDE
5-METHYLINDOLE-3-ALDEHYDE
5-METHYLINDOLE-3-CARBALDEHYDE
5-METHYLINDOLE-3-CARBOXALDEHYDE
5-METHYLINDOLE-3-CARBOXYALDEHYDE
AKOS JY2082657
CBI-BB ZERO/005313
1H-Indole-3-carboxaldehyde, 5-methyl-(9CI)
5-Methylindole-3-carboxaldehyde 98%
5-METHYLINDOLE-3-CARBOXALDEHYDE 98% (HPLC)
5-Methyl-1H-indole-3-carboxaldehyde | [Molecular Formula]
C10H9NO | [MDL Number]
MFCD00022720 | [Molecular Weight]
159.18 | [MOL File]
52562-50-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
2933998090 |
| Hazard Information | Back Directory | [Uses]
5-Methylindole-3-carboxaldehyde was used in the synthesis of novel flexible tripodal ligand derived from 3-methylindole and its mononuclear Zn(II),Cu(II), Ni(II), Hg(II) and Pd(II) complexes. | [Uses]
5-Methyl-1H-indole-3-carbaldehyde (cas# 52562-50-2) is a useful reagent for regio- and enantioselective preparation of N-alkylated indole- and pyrrole-3-carbaldehydes. | [Synthesis]
GENERAL STEPS: Oxalyl chloride (0.3 mL) was slowly added dropwise to cooled (ice bath) N,N-dimethylformamide (DMF, 3 mL) under stirring conditions. The reaction mixture was stirred continuously at 0 °C for 1 hour. Subsequently, a solution of 5-methylindole (4 mmol) dissolved in DMF (1.5 mL) was added slowly dropwise to the above reaction mixture. The resulting mixture was stirred and reacted at room temperature for 5 hours. Upon completion of the reaction, 2N sodium hydroxide solution (2 mL) was added and the mixture was heated to 100 °C and maintained for 10 min. The reaction solution was cooled and extracted with ethyl acetate (3 x 50 mL). The organic phases were combined and washed sequentially with water and saturated brine. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by fast column chromatography with the eluent of ethyl acetate/petroleum ether (3:1, v/v) to finally obtain the purified 5-methyl-1H-indole-3-carbaldehyde. | [References]
[1] European Journal of Medicinal Chemistry, 2013, vol. 65, p. 158 - 167
[2] Patent: EP1932833, 2008, A1. Location in patent: Page/Page column 11; 12
[3] Chemistry - A European Journal, 2016, vol. 22, # 2, p. 716 - 723
[4] Marine Drugs, 2014, vol. 12, # 4, p. 1757 - 1772
[5] European Journal of Medicinal Chemistry, 2012, vol. 52, p. 70 - 81 |
|
|