| Identification | More | [Name]
BGJ398 (NVP-BGJ398) | [CAS]
872511-34-7 | [Synonyms]
BGJ 398
BGJ-398
NVP-BGJ398
BGJ398 (NVP-BGJ398)
3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-[6-[[4-(4-ethylpiperazin-1-yl)phenyl]amino]pyrimidin-4-yl]-1-methylurea
Urea, N'-(2,6-dichloro-3,5-dimethoxyphenyl)-N-[6-[[4-(4-ethyl-1-piperazinyl)phenyl]amino]-4-pyrimidinyl]-N-methyl-
Urea, N'-(2,6-dichloro-3,5-dimethoxyphenyl)-N-[6-[[4-(4-ethyl-1-piperazinyl)phenyl]amino]-4-pyrimidinyl]-N-methyl-, methanesulfonate
3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-[6-[[4-(4-ethylpiperazin-1-yl)phenyl]amino]pyrimidin-4-yl]-1-methylurea NVP-BGJ398
NVP-BGJ398 3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-[6-[[4-(4-ethylpiperazin-1-yl)phenyl]amino]pyrimidin-4-yl]-1-methylurea | [Molecular Formula]
C26H31Cl2N7O3 | [MDL Number]
MFCD22123241 | [MOL File]
872511-34-7.mol | [Molecular Weight]
560.475 |
| Chemical Properties | Back Directory | [Melting point ]
>211°C (dec.) | [Boiling point ]
747.9±60.0 °C(Predicted) | [density ]
1.354 | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO (up to 5 mg/ml) | [form ]
White solid. | [pka]
11.02±0.70(Predicted) | [color ]
White | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. | [InChIKey]
QADPYRIHXKWUSV-UHFFFAOYSA-N | [SMILES]
N(C1C=C(NC2=CC=C(N3CCN(CC)CC3)C=C2)N=CN=1)(C)C(NC1=C(Cl)C(OC)=CC(OC)=C1Cl)=O |
| Hazard Information | Back Directory | [Description]
NVP-BGJ398 (872511-34-7) is a potent and selective pan-FGFR inhibitor (IC50?= 0.9nM, 1.4 nM, 1.0 nM, and 60 nM for FGFR1,2,3,4 respectively).1? It has also been used in a mouse model of Achondroplasia (most common form of dwarfism) to correct pathological hallmarks of this condition. | [Definition]
ChEBI: BGJ-398 is a member of the class of phenylureas that is urea in which a hydrogen attached to one of the nitrogens is replaced by a 2,6-dichloro-3,5-dimethoxyphenyl group, while the hydrogens attached to the other nitrogen are replaced by a methyl group and a 6-{[4-(4-ethylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl group. It is a potent and selective fibroblast growth factor receptor inhibitor. It has a role as a fibroblast growth factor receptor antagonist and an antineoplastic agent. It is an aminopyrimidine, a N-arylpiperazine, a N-alkylpiperazine, a dichlorobenzene and a member of phenylureas. | [Indications]
Infigratinib is approved by the USFDA for the treatment of previously treated patients with advanced or metastatic cholangiocarcinoma carrying FGFR2 fusion or rearrangement. Its inhibitory effect on FGFR helps reduce the proliferation of tumor cells, especially for cholangiocarcinoma patients with abnormal activation of the FGFR pathway. It is used as an oral drug, which provides convenience for patients. | [Brand name]
Truseltiq | [General Description]
Class: receptor tyrosine kinase;
Treatment: cholangiocarcinoma;
Other name: NVP-BGJ398;
Elimination half-life = 34 h;
Protein binding = 96.8% | [Mechanism of action]
Infigratinib works at the biochemical and cellular levels by highly selectively inhibiting the activity of FGFR1, FGFR2, FGFR3, and FGFR4. Activation of the FGFR pathway is associated with the proliferation of malignant cancer cells, therefore, inhibition of FGFR-specific kinases can effectively reduce tumor cell proliferation. | [Side effects]
Side effects of infigratinib may include stomatitis, fatigue, decreased appetite, high blood pressure, and elevated liver enzyme levels. | [Synthesis]
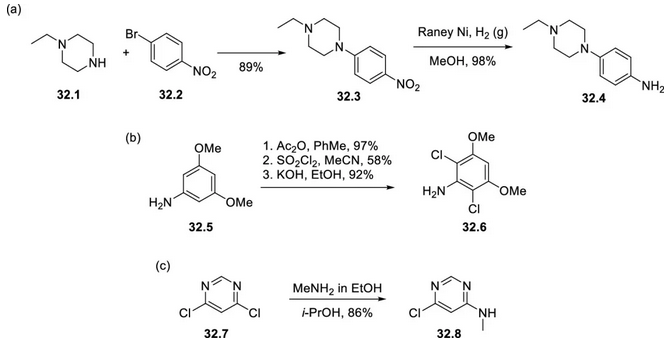
Infigratinib was synthesized in a modular and polymeric manner by combining three building blocks. Piperidine-substituted aniline 32.4 was prepared by SNAr reaction of piperidine 32.1 and bromonitrobenzene 32.2 at 80°C followed by nitro reduction (Figure 1a). 2,6-Dichloro-3,5-dimethoxyaniline (32.6) was obtained from commercially available 3,5-dimethoxyaniline (32.5) in three steps (acylation, chlorination, and hydrolysis) (Figure 1b), and 6-chloro-N-methylpyrimidin-4-amine (32.8) was prepared by SNAr reaction of dichloropyrimidine 32.7 with methylamine (Figure 1c).
Aniline 32.4 and chloropyrimidine 32.8 were SNAr reacted to generate arylmethylamine 32.9, thereby assembling Infigratinib (Figure 2). The aniline derivative 32.6 was converted in situ to the corresponding isocyanate and combined with 32.9 to form a urea bond, completing the synthesis of Infigratinib.
 | [target]
FGFR1 | [IC 50]
FGFR1~4 IC50 = 0.9, 1.4, 1.0, and 60
nM; VEGFR2 IC50 = 180 nM | [References]
1) Guagnano?et al.?(2011),?Discovery of 3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-(6-{[4-(4-ethyl-1-piperazinyl)phenyl]amino}-4-pyrimidinyl)-1-methylurea (NVP-BGJ398),?a Potent and Selective Inhibitor of the Fibroblast Growth Factor Receptor Family of Receptor Tyrosine Kinase?; J. Med. Chem.?54?7066
2) Komla-Ebri?et al.?(2016),?Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model; J. Clin. Invest.?126?1871 |
|
|