[Synthesis]
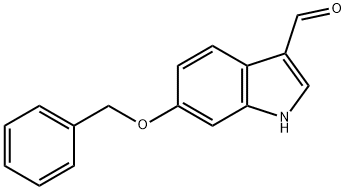
General procedure for the synthesis of 6-benzyloxyindole-3-carboxaldehyde from 6-benzyloxyindole and N,N-dimethylformamide: A three-necked round-bottomed flask equipped with a nitrogen inlet and a thermocouple was charged with N,N-dimethylformamide (DMF, 75 mL). The flask was cooled to an internal temperature of +2.8 °C using an ice water bath. Phosphoryl chloride (12.7 mL, 137.2 mmol, 1.25 eq.) was slowly added dropwise, controlling the internal temperature to be below 5 °C (1 hour elapsed time). After dropwise addition, 6-benzyloxyindole (25.0 g, 109.7 mmol, 1.0 eq.) was added in batches of 1 g at a time, keeping the internal temperature below 5 °C (1 hour elapsed time). The resulting dark-colored solution was slowly warmed to room temperature (RT) and maintained for 1 hour. An aqueous solution (250 mL) of sodium hydroxide (50 g) was prepared in a Morton flask and cooled to an internal temperature of 2.0°C. The reaction mixture was slowly poured into the cold sodium hydroxide solution under vigorous stirring, controlling the internal temperature between 20-30°C. A light beige solid (final pH 14) was separated. The slurry was slowly heated to an internal temperature of 70°C and held under a light nitrogen purge for 10-15 minutes to remove precipitated dimethylamine, followed by continued heating to an internal temperature of 90°C and holding for 15 minutes. After cooling to room temperature, the slurry was diluted with water (40 mL) and the solids were collected by filtration. The filter cake was washed with water (2 x 100 mL) and dried in a vacuum oven at 50 °C and 23 inches Hg pressure with a slight nitrogen purge to constant weight to give 6-benzyloxyindole-3-carboxaldehyde (27.3 g) as a beige/tan solid. The product can be used directly without further purification, or if in bulk form, it needs to be ground into a fine powder before the next step.1H NMR (400 MHz, DMSO-d6) δ: 11.94 (br, s), 9.87 (s, 1H), 8.15 (s, 1H), 7.96 (d, J = 8.5 Hz, 1H), 7.47 (d, J = 7.2 Hz, 2H ), 7.40 (app t, J = 7.4 Hz, 2H), 7.33 (t, J = 7.0 Hz, 1H), 7.08 (s, 1H), 6.95 (d, J = 8.5 Hz, 1H), 5.15 (s, 2H). 13C NMR (101 MHz, DMSO-d6): 184.7, 155.7, 137.9, 137.8, 137.2, 128.4, 127.7, 127.6, 121.4, 118.25, 118.23, 112.4, 96.9, 69.5. IR (KBr) (cm-1): 1634 (s), 1526 (m), 1426 (s, 2H). 1526 (m), 1426 (m), 1385 (m), 1159 (m). HRMS (ESI) (m/z): Calcd for C16H13NO2 [M + H]+: 252.1019. Found: 252.1025. |
[References]
[1] Journal of Medicinal Chemistry, 2012, vol. 55, # 23, p. 10700 - 10715
[2] Journal of the Chemical Society, 1958, p. 3493,3494
[3] Synlett, 2003, # 10, p. 1533 - 1535
[4] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 9, p. 2571 - 2574
[5] Journal of Organic Chemistry, 2018, vol. 83, # 7, p. 3928 - 3940 |