Benzamid
|
|
Benzamid Eigenschaften
- Schmelzpunkt:
- 125-128 °C (lit.)
- Siedepunkt:
- 228°C
- Dichte
- 1.341
- chüttdichte
- 550kg/m3
- Dampfdruck
- 0.000056 hPa (20 °C)
- Brechungsindex
- 1.5323 (estimate)
- Flammpunkt:
- 180°C
- storage temp.
- Store below +30°C.
- L?slichkeit
- ethanol: soluble50mg/mL, clear to very slightly hazy, colorless to light yellow
- Aggregatzustand
- Crystalline Powder
- pka
- 13.0(at 25℃)
- Farbe
- White to almost white
- S?ure-Base-Indikators(pH-Indikatoren)
- 6.9
- PH
- 6.9 (H2O)(saturated solution)
- Wasserl?slichkeit
- 1.35 g/100 mL (20 ºC)
- Merck
- 14,1060
- BRN
- 385876
- Stabilit?t:
- Stable. Combustible. Incompatible with strong oxidizing agents.
- LogP
- 0.640
- CAS Datenbank
- 55-21-0(CAS DataBase Reference)
- NIST chemische Informationen
- Benzamide(55-21-0)
- EPA chemische Informationen
- Benzamide (55-21-0)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn | ||
|---|---|---|---|
| R-S?tze: | 22-68 | ||
| S-S?tze: | 22-24/25-36/37 | ||
| WGK Germany | 1 | ||
| RTECS-Nr. | CU8700000 | ||
| Selbstentzündungstemperatur | >500 °C | ||
| TSCA | Yes | ||
| HS Code | 29242995 | ||
| Giftige Stoffe Daten | 55-21-0(Hazardous Substances Data) | ||
| Toxizit?t | LD50 orally in Rabbit: 1125 mg/kg |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Benzamid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.S24/25:Berührung mit den Augen und der Haut vermeiden.
Aussehen Eigenschaften
C7H7NO; Benzolcarboxamid, Benzoesäureamid. Farbloser, feinkristalliner Feststoff.Gefahren für Mensch und Umwelt
Gesundheitsschädlich beim Verschlucken.Brennbar.
LD50 (oral, Maus): 1160 mg/kg
Schutzma?nahmen und Verhaltensregeln
Geeignete Schutzhandschuhe als kurzzeitiger Staubschutz.Verhalten im Gefahrfall
Staubentwicklung vermeiden.Trocken aufnehmen. Der Entsorgung zuführen. Nachreinigen.
Kohlendioxid, Wasser, Pulver.
Brennbar. Im Brandfall können nitrose Gase entstehen.
Erste Hilfe
Nach Hautkontakt: Mit reichlich Wasser abwaschen.Nach Augenkontakt: Mit reichlich Wasser bei geöffnetem Lidspalt mindestens 10 Minuten ausspülen. Sofort Augenarzt hinzuziehen.
Nach Einatmen: Frischluft.
Nach Verschlucken: Reichlich Wasser trinken lassen. Erbrechen auslösen. Sofort Arzt hinzuziehen.
Nach Kleidungskontakt: Kontaminierte Kleidung entfernen.
Ersthelfer: siehe gesonderten Anschlag
Sachgerechte Entsorgung
Gelöst in z.B. Aceton als halogenfreie, organische Lösemittelabfälle.Beschreibung
Benzamide appears as off-white crystals or powder. It is combustible and incompatible with strong oxidising agents and strong bases. On combustion and thermal decomposition, it emits nitrogen oxides, carbon monoxide, and carbon dioxide.Benzamide is a carbonic acid amide of benzoic acid. Benzamide exhibits an angle of about 15º with the plane of the amide group; this shows that benzamide molecule is not flat. The rotation of the amide group relative to the aromatic ring may result from the repulsion interaction between the hydrogen atoms of the amide group and those of the aromatic ring.
Chemische Eigenschaften
Benzamide is a combustible, colorless to beige, off-white, crystalline solid; freezing/melting point=132-133° C. It is slightly soluble in water, and soluble in many organic solvents.
Benzamide was used to study the mechanism of photocatalytic decomposition of aqueous solution of acetic acid, acetamide and acetonitrile in the presence of semiconductors. It was used to develop a robust screening method to study biotransformations using (+)-γ-lactamase enzyme.
Verwenden
Organic synthesis.Benzamide on radioiodination by different labeling procedures results in large-scale production of radioiodinated benzamides having potential therapeutic application for patients with metastatic malignant melanoma.
Definition
ChEBI: An aromatic amide that consists of benzene bearing a single carboxamido substituent. The parent of the class of benzamides.synthetische
Take a mixture of 5 ml concentrated ammonia and 5 ml water in a conical flask with a well-fitting cork. Add 2 ml (2.4 g.) benzoyl chloride, cork the flask and shake vigorously. Heat generates due to the reaction, hence hold the cork securely during shaking. After 15 min not even a trace of oily benzoyl chloride remains. Filter the fine flakes, wash with cold water and recrystallise from hot water: yield, 1-5 g. Colourless crystals of benzamide.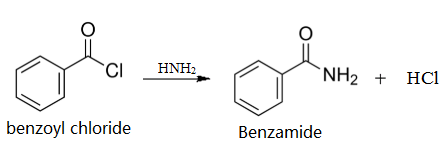
Preparation of benzamide from benzoyl chloride
Allgemeine Beschreibung
White powder.Air & Water Reaktionen
Insoluble in water.Reaktivit?t anzeigen
Benzamide reacts with azo and diazo compounds to generate toxic gases. Forms flammable gases with strong reducing agents. Mixing with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. Combustion generates toxic mixed oxides of nitrogen (NOx).Hazard
Depresses the central nervous system; toxic.Brandgefahr
Flash point data for Benzamide are not available, however Benzamide is probably combustible.Clinical Use
Benzamide on radioiodination by different labeling procedures results in large-scale production of radioiodinated benzamides having potential therapeutic application for patients with metastatic malignant melanoma.m?gliche Exposition
Benzamide is used in organic synthesis.l?uterung methode
Crystallise it from hot water (about 5mL/g), EtOH or 1,2-dichloroethane, and dry it in air. It has also been crystallised from dilute aqueous NH3, H2O, Me2CO, then *C6H6 using a Soxhlet extractor. Dry it in an oven at 110o for 8hours and store in a desiccator over 99% H2SO4. [Bates & Hobbs J Am Chem Soc 73 2151 1951, Beilstein 9 IV 725.]Benzamid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Glass-lined reaction pot
Benzoylchlorid
Diammoniumcarbonat
Methylbenzimidathydrochlorid
Trimethylorthobenzoat
Downstream Produkte
Benzoylisocyanat
(3S,4R)-4-Acetoxy-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]azetidin-2-one
Methylanthranilat
Bifenthrin
Butyl-2-[[3-[[(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)amino]carbonyl]-2-hydroxy-1-naphthyl]azo]benzoat
Methyl-2-[[3-[[(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)amino]carbonyl]-2-hydroxy-1-naphthyl]azo]benzoat
2-(5-METHYL-2-PHENYL-1,3-OXAZOL-4-YL)ACETIC ACID
Procainamidhydrochlorid
Benzonitril
Brotianid
4-Piperidon
Cisaprid
2-Phenylindol
N-Benzylideneamine
2-MORPHOLIN-4-YL-1,3-THIAZOL-4(5H)-ONE
SUCCINALDEHYDIC ACID
N,N'-(9,10-Dihydro-9,10-dioxoanthracen-1,4-diyl)bisbenzamid
Benzamid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 477)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +8613288715578 |
sales@hbmojin.com | China | 12580 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2988 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 |
figo.gao@foxmail.com | China | 8497 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Changzhou Carman Chemical Co., Ltd. | 15061933924 |
CHINA | 514 | 58 | |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63687 | 58 |
55-21-0(Benzamid)Verwandte Suche:
p-Aminobenzamid
Polyamide
p-Hydroxybenzamid
2,6-Difluorbenzamid
4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid
Benzohydrazid
ACETIC ACID
Benzamidoxim
ASCORBICACID
4-Nitrobenzamid
5-[2-Chlor-4-(trifluormethyl)phenoxy]-N-(methylsulfonyl)-2-nitrobenzamid
3,5-Dinitrobenzamid
Cisaprid
4-Fluorbenzamid
m-Aminobenzamid
Formamid
o-Amino-N-(2-chlor-3-pyridyl)benzamid
Benzamid
- ai3-01031
- amidkyselinybenzoove
- amidkyselinybenzoove(czech)
- benzenecarboxamide
- Carbonamide
- phenylcarboxamide
- Phenylcarboxyamide
- BENZAMIDE, SUBLIMED, ZONE-REFINED, 99.9%
- BENZAMIDE, SUBLIMED, 99.5+%
- BENZAMIDE CRYSTALLINE
- BENZAMIDE INHIBITOR OF POLY (AD
- BENZAMIDE 98+%
- BENZOYLAMIDE
- BENZOIC AMIDE
- BENZAMIDE
- BenzamideForSynthesis
- Benzamide-ring-13C6
- BENZOICACIDAMINOSALT
- Benzamide Zone Refined (number of passes:20)
- BENZAMIDE pure
- Benzamide,99%
- Benzamide Zone Refined
- BenzaMide, 99% 100GR
- BenzaMide, 99% 500GR
- BenzaMide, 99% 5GR
- BENZAMIDE FOR SYNTHESIS 250 G
- BENZAMIDE FOR SYNTHESIS 25 KG
- BENZAMIDE FOR SYNTHESIS 5 G
- BenzaMide purified by subliMation, >=99.5%
- BenzaMide 0.25
- Benzamide Vetec(TM) reagent grade, 98%
- NSC 3114
- Cefaclor Impurity I
- Benzamide >
- Benzamide ISO 9001:2015 REACH
- Benzamide 500GM
- Benzamide 55-21-0
- Benzamide (SQ), Qualigens
- BENZOIC ACID AMIDE
- Benzamid
- Cefaclor Impurities 15
- Benzamide 98% For Synthesis
- Benzamide, 10 mM in DMSO
- 55-21-0
- C6H4CONH2
- Organic Building Blocks
- PARP
- PARP Regulators
- Building Blocks
- Amides
- BioChemical
- Cell Biology
- Cell Signaling and Neuroscience
- Apoptosis and Cell Cycle
- Carbonyl Compounds
- Amides
- B
- Bioactive Small Molecules







