|
|
| | (S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II) Basic information | | Reaction |
| Product Name: | (S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II) | | Synonyms: | -1,2-diphenylethanediamine(chloro);-N-(p-Toluenesulfonyl);(S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II);Chloro{[(1S,2S)-(+)-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido}(p-cymene)ruthenium(II);[N-[(1S,2S)-2-(Amino-kN)-1,2-diphenylethyl]-4-methylbenzenesulfonamidato-kN]chloro[(1,2,3,4,5,6-n)-1-methyl-4-(1-methylethyl)benzene]ruthenium;Chloro{[(1S,2S)-(+)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido}(p-
cymene)ruthenium(II), min. 95% RuCl[(S,S)-Tsdpen](p-cymene);Chloro{[(1S,2S)-(+)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido}(p-cymene)ruthenium(II), min. 95%;Chloro{[(1S,2S)-(+)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido}(p-cymene)ruthenium(II), min. 95% RuCl[(S,S)-Tsdpen](p-cymene) | | CAS: | 192139-90-5 | | MF: | C31H30ClN2O2RuS | | MW: | 631.17 | | EINECS: | | | Product Categories: | organometallic complex;Ru | | Mol File: | 192139-90-5.mol | 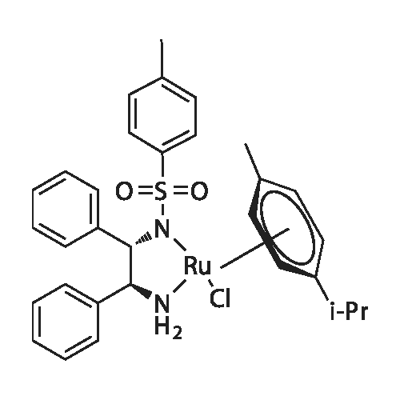 |
| | (S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II) Chemical Properties |
| Melting point | >175 °C | | alpha | 178o (C=0.5 IN CHLOROFORM) | | storage temp. | 2-8°C | | solubility | soluble in Chloroform, DCM, Ethyl Acetate | | form | solid | | color | yellow to dark brown | | optical activity | [α]20/D +178°, c = 0.5 in chloroform | | Sensitive | air sensitive | | InChIKey | DCJKLKCGNMDXFR-MNPNNRAMNA-M | | SMILES | [Cl-][Ru+2]123456(N[C@@H](C7C=CC=CC=7)[C@H](C7C=CC=CC=7)[N-]1S(C1C=CC(C)=CC=1)(=O)=O)C1(C)C2=C3C4(C(C)C)=C5C6=1 |&1:3,10,r| |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 24/25 | | WGK Germany | 3 | | HS Code | 28439000 |
| | (S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II) Usage And Synthesis |
| Reaction | This catalyst has shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydoxy, siloxy, carbonyl, ester, amide or thioester.

| | Chemical Properties | Solid | | Uses | Catalyst involved in:
- Asymmetric transfer hydrogenation of imines and ketones
- Intramolecular asymmetric reductive amination
- Tandem hydroformylation / hydrogenation of terminal olefins
Reactant involved in studies of thermal decomposition of areneruthenium chiral amido-amine alkyl complexes | | Uses | Catalyst involved in: ? Asymmetric transfer hydrogenation of imines and ketones ? Intramolecular asymmetric reductive amination ? Tandem hydroformylation / hydrogenation of terminal olefins Reactant involved in studies of thermal decomposition of arenerut | | General Description | RuCl(p-cymene)[(S,S)-Ts-DPEN] is a chiral diamine ligand complexed with ruthenium, which can be used for the asymmetric transfer hydrogenation of a variety of imines. |
| | (S,S)-N-(p-Toluenesulfonyl)-1,2-diphenylethanediamine(chloro)(p-cymene)ruthenium(II) Preparation Products And Raw materials |
|