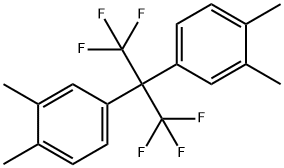
2,2-Bis(3,4-dimethylphenyl)hexafluoropropane synthesis
- Product Name:2,2-Bis(3,4-dimethylphenyl)hexafluoropropane
- CAS Number:65294-20-4
- Molecular formula:C19H18F6
- Molecular Weight:360.34
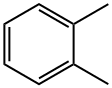
95-47-6

684-16-2

65294-20-4
In a 2000 mL three-necked flask, 1000 g of tetrahydrofuran (THF), 424 g of o-xylene, and 60 g of p-toluenesulfonic acid (p-TsOH) were added, and the reaction temperature was controlled to be below 40°C. The reaction was carried out by a slow pass of 400 g of hexafluoroacetone gas. 400 g of hexafluoroacetone gas was slowly passed. After heating and refluxing the reaction for 12 hours, it was cooled to room temperature. The reaction mixture was washed with saturated sodium bicarbonate (NaHCO3) aqueous solution to pH 7-8. 500 g of toluene was added, partitioned and the organic phase was washed with deionized water. The washed organic phase was concentrated to give a solid crude product. The crude product was recrystallized by dissolving in 1200 g of ethanol. The wet product was dried under vacuum at 40 ℃ for 12 hours to obtain 670 g of white crystal product with 93.1% yield. The product was analyzed by gas chromatography (GC), and the purity was 99.6%, the content of single impurity was less than 0.1%, and the content of all metal ions was less than 1 ppm.
Yield:65294-20-4 98.7%
Reaction Conditions:
with hydrogen fluoride at 100; under 7980.54 Torr; for 13 h;Autoclave;Concentration;Temperature;
Steps:
1 Example 3
Equipped with magnetic stirring, condenser, oil bath heating,Water separator 500mL three-necked flask was added hexafluoroacetone trihydrate 82g (0.372mol), o-xylene 90g (0.85mol), layered,Stirring, the reaction mixture was milky white, warmed to reflux temperature,Water separator out of water (water separator added about 40g o-xylene),Continue to heat reflux to the basic water separation, the reaction flask liquid colorless and transparent,O-xylene and hexafluoroacetone monohydrate mixture;The middle and lower water separator 16g, 12.3mL, density 1.30g / cm3,A small amount of hexafluoroacetone trihydrate separated.0.5 L of the above-obtained mixture of o-xylene and hexafluoroacetone monohydrate was addedThe autoclave, adding hydrogen fluoride hydrofluoric acid 111g (5.58mol), open stirring,Heated to 100 ° C for about 13h, the reaction pressure of about 8.9atm, after the reaction,Cooling, relieving pressure, purging nitrogen three times, exhaust gas water, caustic absorption,Open the kettle, the reaction mixture was poured into ice water with stirring, precipitated solid, filtered,About 100 g of a yellow solid was obtained. To invest in hexafluoroacetone trihydrate,The crude yield is about 75%.After the crude product is dried, it is recrystallized from isopropanol,86.4 g of white crystalline powder was obtained,The content of 2,2-bis (3,4-dimethylphenyl) hexafluoropropane is about 98.7%.
References:
CN106699504,2017,A Location in patent:Paragraph 0023; 0024; 0025; 0026; 0027; 0028-0038
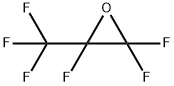
116-16-5
184 suppliers
$15.00/25G

95-47-6
616 suppliers
$10.00/25g

65294-20-4
195 suppliers
$5.00/1g