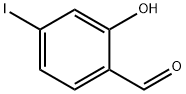
2-HYDROXY-4-IODO-BENZALDEHYDE synthesis
- Product Name:2-HYDROXY-4-IODO-BENZALDEHYDE
- CAS Number:38170-02-4
- Molecular formula:C7H5IO2
- Molecular Weight:248.02

50-00-0

533-58-4

38170-02-4
Step 1: Preparation of 2-hydroxy-4-iodobenzaldehyde Magnesium chloride (MgCl2, 19.5 g, 204 mmol) was added batchwise to a frozen solution of commercially available 2-iodophenol (30 g, 136 mmol) in acetonitrile (ACN) while keeping the reaction temperature below 10 °C. Paraformaldehyde (28.6 g, 954 mmol) and triethylamine (TEA, 76 mL, 545 mmol) were subsequently added and the reaction was exothermic to 15 °C. The reaction mixture was heated to 72 °C and maintained for 2 hours. Upon completion of the reaction, it was cooled to room temperature, poured into saturated aqueous ammonium chloride solution (500 mL) and extracted with ethyl acetate (2 x 150 mL). The organic phases were combined and washed sequentially with aqueous sodium bicarbonate (NaHCO3) (2 × 150 mL), aqueous 1N hydrochloric acid (HCl) (2 × 150 mL) and brine (2 × 150 mL). The organic layer was dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography (silica gel, 5% ethyl acetate/hexane). The target fractions were collected, combined and the solvent was removed under reduced pressure to give 2-hydroxy-4-iodobenzaldehyde (27 g, 79% yield) as a yellow solid. The salicylaldehyde derivative was of suitable purity and could be used directly in the subsequent reaction.

50-00-0
893 suppliers
$10.00/25g

533-58-4
380 suppliers
$6.00/5g

38170-02-4
70 suppliers
$125.00/250mg
Yield: 79%
Reaction Conditions:
with triethylamine;magnesium chloride in acetonitrile at 10 - 72; for 2 h;
Steps:
3.1
4-(nitrooxy)butyl (2R)-7-benzyl-6-chloro-2-(trifluoromethyl)-2H-chromene-3- carboxylate; Step 1; Preparation of 2-hydroxy-4-iodobenzaldehyde; [0168] To a chilled solution of commercially available 2-iodophenol (30 g, 136 mmole) in ACN was added MgCl2 (19.5 g, 204 mmole) portion-wise while maintaining the temperature below 10 0C, followed by paraformaldehyde (28.6 g, 954 mmole) and TEA (76 mL, 545 mmole) producing a 15 °C exotherm. The solution was heated to 72 0C for 2 h. The reaction was cooled to room temperature and poured into Saturated aqueous Ammonium Chloride (500 mL), extracted with ethyl acetate (2 X 150 mL). The combined organic phases were washed with aqueous NaHCO3 solution (2 X 150 mL), aqueous IN HCl solution (2 X 150 mL), and brine (2 X 150 mL), dried over Na2SO4, filtered and concentrated in vacuo. The crude material was subjected to flash chromatography (Silica, 5% Ethyl acetate/ Hexane). Desired fractions were collected and combined, removed solvent in vacuo producing the ethyl ester (27 g, 79%) as a yellow solid. This salicylaldehyde was of suitable purity to use without further purification.
References:
PHARMACIA & UPJOHN COMPANY LLC WO2006/40676, 2006, A1 Location in patent:Page/Page column 51

50-00-0
893 suppliers
$10.00/25g

626-02-8
303 suppliers
$9.00/5g

38170-02-4
70 suppliers
$125.00/250mg

50-00-0
893 suppliers
$10.00/25g

626-02-8
303 suppliers
$9.00/5g

38169-97-0
25 suppliers
inquiry

38170-02-4
70 suppliers
$125.00/250mg

67-66-3
0 suppliers
$33.60/500ml

626-02-8
303 suppliers
$9.00/5g

38170-02-4
70 suppliers
$125.00/250mg
18179-39-0
149 suppliers
$8.00/1g

38170-02-4
70 suppliers
$125.00/250mg
